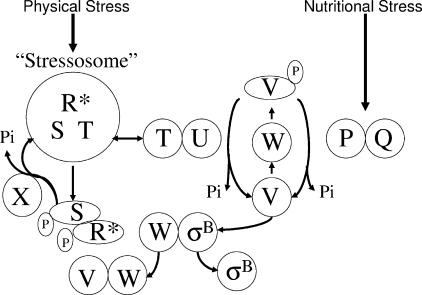
FIG. 1.
Model of σB control. As depicted in the lower portion of the diagram, σB is normally inactive, complexed with the anti-σB protein RsbW (W). σB is freed from RsbW when a release factor, RsbV (V) binds RsbW in lieu of σB. In the absence of stress, RsbV is unable to trigger σB release due to an RsbW-catalyzed phosphorylation. RsbV is dephosphorylated by one of two stress-responsive phosphatases that uniquely respond to physical or nutritional stress. The nutritional stress phosphatase, RsbP (P), requires a coexpressed protein, RsbQ (Q), for activity. The RsbQ/RsbP trigger is unknown, but its activation coincides with conditions that cause a drop in ATP. The physical stress phosphatase, RsbU (U), is activated by a second protein, RsbT (T), that is ordinarily bound to a negative regulator, RsbS (S), in a large (>106-Da) complex (stressosome) formed from RsbR and a family of paralogous proteins (R*). Following exposure to physical stress, an unknown mechanism allows RsbR and RsbS to become phosphorylated by RsbT. This frees RsbT to activate the RsbU phosphatase. RsbR-P and RsbS-P are dephosphorylated and reactivated by RsbX (X), a phosphatase whose levels increase following σB activation. This model is based on references given in the text.