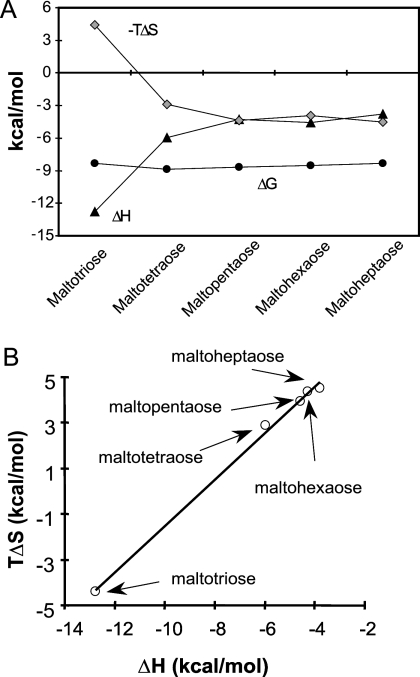
FIG. 4.
Enthalpy-entropy compensation. (A) Plots of ΔG (•), ΔH (▴), and −TΔS (⧫) versus maltodextrins. The data show two trends of enthalpy changes depending on the number of glucose units; one is the rapid change from maltotriose to maltotetraose, showing the effect of extra glucose moiety binding, and the other is the lack of change from maltopentaose to maltoheptaose, indicating that the extra glucose no longer contacts the protein. (B) Plot of ΔH versus TΔS for all the maltodextrins with a slope of 1, suggesting that there is an enthalpy-entropy compensation effect that results in minimal change in the total binding free energy.