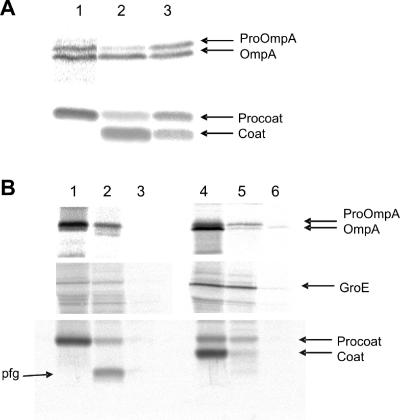
FIG. 3.
Expression of procoat protein −6C inhibits the leader peptidase. E. coli K38 cells bearing a plasmid expressing procoat protein −6C, −7C, or −12C were grown in minimal M9 medium and induced for 2 h with 1 mM IPTG. [35S]methionine was added for 1 min, and the sample was immediately chilled by mixing it with ice-cold medium. (A) The cells were acid precipitated and analyzed to determine their processing of proOmpA (upper panel) and procoat protein (lower panel) by immunoprecipitation, SDS-PAGE, and phosphorimaging. The results for cells expressing procoat protein −6C (lane 1), procoat protein −7C (lane 2), and procoat protein −12C (lane 3) are shown. The positions of proOmpA, OmpA, procoat protein, and coat protein are indicated by arrows. (B) To analyze the membrane translocation of the proteins, the cells were converted to spheroplasts, and 1 mg/ml proteinase K was added to the outside of the cells for 1 h on ice (lanes 2 and 5). The results for cells expressing procoat protein −6C (lanes 1 to 3) and procoat protein −7C (lanes 4 to 6) are shown. Cells without proteinase K (lanes 1 and 4) and cells treated with TritonX-100 and proteinase K (lanes 3 and 6) were used as controls. The cytoplasmic GroE protein was analyzed to show that the spheroplasts remained intact during proteolysis.