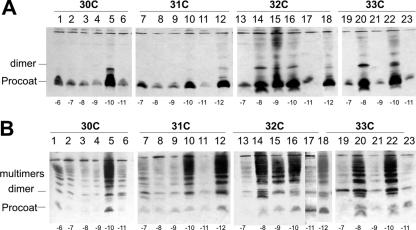
FIG. 8.
Oligomerization of H5 procoat protein double-cysteine mutants. E. coli BL-21 cells bearing plasmids expressing procoat protein H5 with two additional cysteine residues at the positions indicated below were induced for 4 h (A) or were induced for 4 h and incubated with 1 mM copper phenanthroline for 10 min (B). The samples were acid precipitated and analyzed by nonreducing SDS-PAGE and Western blotting with anti-coat protein. One cysteine residue was at position 30 (lanes 1 to 6), 31 (lanes 7 to 12), 32 (lanes 13 to 18), or 33 (lanes 19 to 23). The second cysteine was at position −6 (lane 1), −7 (lanes 2, 7, 13, and 19), −8 (lanes 3, 8, 14, and 20), −9 (lanes 4, 9, 15, and 21), −10 (lanes 5, 10, 16, and 22), −11 (lanes 6, 11, 17, and 23), or −12 (lanes 12 and 18).