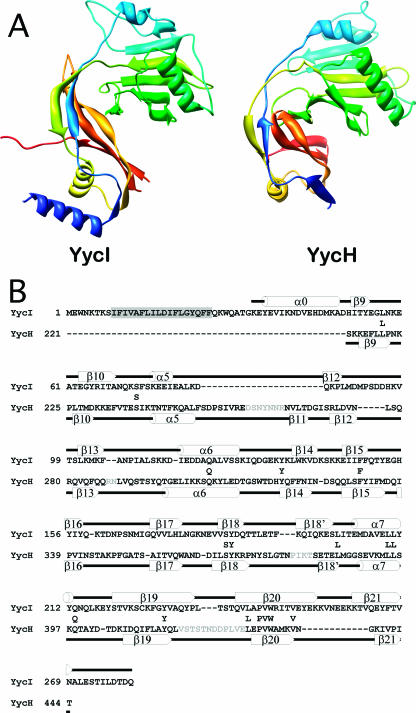
FIG. 2.
Comparison of a YycI subunit and a YycH monomer. (A) The two structures are shown in the same orientation after structural alignment colored in blue-green-yellow-orange-red so that corresponding structural elements are in similar colors. Only the fragment of YycH that aligns with YycI is shown for clarity. (B) A structure-based sequence alignment of YycI and YycH was generated using the DALI server. For accuracy, individual domains were aligned separately and then assembled into a single alignment. Residues conserved between YycH and YycI are shown between the two sequences. Secondary structure elements for YycI and YycH are shown above and below each sequence, respectively, and were labeled as previously defined for YycH (27). One strand in YycH located between β18 and β19 was not previously labeled and was therefore named β18′. The N-terminal helix in YycI does not have a counterpart in YycH and was hence named α0. The putative transmembrane domain in YycI is highlighted in gray. Sequences in YycH that were not resolved are in gray.