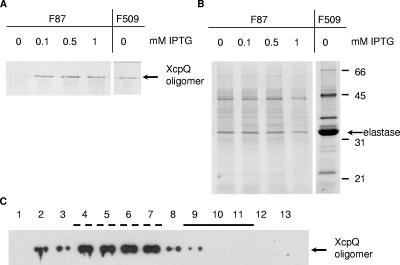
FIG. 3.
Characterization of the nonfunctional hybrid protein XcpQF87. The xcpQ mutant PAN1 with pM2 encoding the nonfunctional hybrid protein XcpQF87 was grown to an optical density at 660 nm of 2.3, after which IPTG was added at the concentrations indicated above the lanes, and growth was continued for 2 h. PAN1(pM28) producing the active fusion protein XcpQF509 was grown without IPTG. To determine the production of XcpQ oligomers, cell envelopes were isolated and subjected to SDS-PAGE and Western blotting with polyclonal antibodies directed against XcpQ (A). To determine the functionality of XcpQ hybrids, secreted proteins were precipitated with 5% TCA and separated by SDS-PAGE (B). The position of the major protein secreted via the type II pathway, elastase, and the positions of the molecular mass standard proteins (in kDa) are indicated on the right. (C) Cellular localization of XcpQF87. A cell envelope preparation of P. aeruginosa PAN1(pM2) expressing XcpQF87 was applied to a 30 to 55% sucrose gradient, which was centrifuged and fractionated. Samples from all fractions were analyzed by SDS-PAGE, and proteins were transferred to a nitrocellulose membrane and visualized by immunodetection using antibodies against XcpQ and enhanced chemiluminescence for detection. The fractions that represented the inner membrane (solid line) and outer membrane (dashed line) were identified based on NADH oxidase activity and the presence of OprF, respectively. The position of the XcpQ oligomers is indicated on the right.