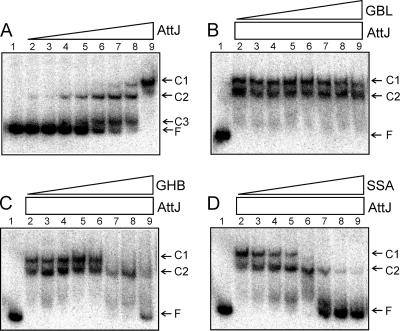
FIG. 2.
(A) EMSA using His-AttJ and radiolabeled attKLM promoter DNA. The positions of the three shifted protein-DNA complexes (C1, C2, and C3) and free DNA (F) are marked. Lane 1 represents free DNA with no added protein. AttJ protein was provided at concentrations of 0.3, 1, 3, 10, 30, 100, 300, and 1,000 nM (lanes 2 to 9). In panels B to D, AttJ was provided at a final concentration of 1,000 nM, and GBL (C), GHB (D), or SSA (E) was provided at concentrations of 10 μM, 30 μM, 100 μM, 300 μM, 1 mM, 3 mM, 10 mM, and 30 mM (lanes 2 to 9, respectively). His6-AttJ was overproduced by using strain BL21(DH3)/pYC160. After lysis and clarification of a 500-ml culture, the soluble fraction was diluted 10-fold with 50 mM phosphate buffer (pH 7.4; 100 mM NaCl, 10% glycerol) and added to a TALON cobalt affinity column (10-ml bed volume) (BD Biosciences). Bound proteins were washed with 40 ml of buffer A (50 mM phosphate buffer [pH 7.4], 300 mM NaCl, 10% glycerol) and eluted using 20 ml of buffer B (buffer A supplemented with 200 mM imidazole). Fractions containing His-AttJ proteins were pooled and dialyzed against 500 ml of phosphate buffer overnight at 4°C. A 217-nucleotide DNA fragment containing the attKLM promoter was amplified by PCR using the oligonucleotides PattKLM-F1 and PattKLM-R1. The resulting PCR products were digested with EcoRI and end labeled using [α-32P]dATP. Binding reactions and size fractionation of the samples were performed according to a previously published protocol (24).