Abstract
Aim
To obtain a better understanding of nociceptive processing in patients with oesophagitis.
Patients and methods
Eleven patients with grade B oesophagitis were compared with an age and sex matched group of 16 healthy subjects. A probe was positioned in the lower part of the oesophagus. After preconditioning of the tissue, painful mechanical stimuli were applied as distensions with a bag using an impedance planimetric method. Distensions were done before and after pharmacological impairment of distension induced smooth muscle contractions. Thermal stimulation was performed by recirculating water at 1 and 60°C in the bag. The area under the temperature curve (AUC) represented caloric load. The referred pain area (being a proxy for the central pain mechanisms) to the mechanical stimuli was drawn at maximum pain intensities.
Results
Patients were hyposensitive to mechanical stimuli, as assessed by the distending volume (F = 8.1, p = 0.005). After relaxation of smooth muscle with butylscopolamine, the difference between the two groups was more evident (F = 27.4, p<0.001). AUC for cold stimulation was 1048.6 (242.7) °C×s in controls and 889.8 (202.6) °C×s in patients (p = 0.5). For heat stimuli, AUC values were 323.3 (104.1) and 81.3 (32.3) °C×s in controls and patients, respectively (p = 0.04). The referred pain area to the mechanical stimulations was larger and more widespread in patients (49.3 (6.2) cm2 compared with controls 23.9 (7) cm2; p = 0.02).
Conclusions
The data indicate that peripheral sensitisation of heat sensitive receptors and pathways combined with facilitation of central pain mechanisms may explain the symptoms in patients with oesophagitis.
Keywords: oesophagitis, experimental pain, acid, sensitisation
Gastro‐oesophageal reflux disease (GORD) is very common in the general population, with 30% of Europeans reporting heartburn and/or acid regurgitation in the previous 12 months.1 However, symptoms in reflux disease are highly variable and poorly understood, introducing bias in epidemiological surveys and treatment protocols. Thus in patients with GORD and in particular in those with erosive disease, no simple relation seems to exist between symptoms and severity of disease.2,3,4 Although treatment with proton pump inhibitors (PPI) is very effective, many patients continue to have symptoms, despite treatment,5,6 and in a recent study 50% of patients continued to have pathological reflux despite effective symptom control with PPI.7 Therefore, further studies are needed to increase our understanding of the symptoms and pain mechanisms in GORD.
The nerves mediating conscious sensations from the oesophagus have free endings and travel mainly with sympathetic nerves. All histological specified layers of the organs seem to be innervated by sensory afferents although density and specificity seem to differ in individual layers. Thus afferents mediating chemical sensations are mainly localised in the mucosa whereas mechanosensitive afferents are mostly prevalent in muscle layers.8,9 Innervation of the oesophagus is more complex than the remaining parts of the gut as the upper third is innervated by somatic afferents and, furthermore, sensations may to some degree be mediated by non‐spinal afferents.10 In the central nervous system (CNS), oesophageal afferents converge on a large scale with neurones receiving input from superficial and deep somatic tissue resulting in referred pain to somatic structures.11 Increased acid exposure of the oesophagus may lead to sensitisation of the nerves resulting in plastic changes not only on peripheral nerves but also in the CNS.12 Such changes are mainly documented in animal studies although recent work in humans has provided evidence that symptoms related to the oesophagus may also be explained by peripheral and/or central sensitisation and neuroplastic changes.13,14
In clinical work, characterisation of pain is confounded by many other symptoms caused by the diseases, such as complaints relating to psychological, cognitive, and social aspects of the illness. Moreover, patients are typically treated with different therapeutic interventions which may cause side effects such as changes in gut motor function. All such confounders can influence the perception of pain and other symptoms making the assessment in clinical studies difficult. One possible way of overcoming this problem is to use experimental human pain models. In these models the investigator can standardise the experimental conditions. Pain “input” (for example, the nature, localisation, intensity, and duration of the stimulus) can be controlled and thus will provide reproducible measures of “output” (for example, psychophysical, behavioural, or neurophysiological response).15 Some experiments used experimental stimuli to evoke pain in patients with GORD and to obtain a better understanding of symptoms.16,17,18 However, some of these previous studies were flawed by the methods used for mechanical stimulation, resulting in errors related to the deformation field and erroneous conclusions.15 Hence methods where distensions are controlled with respect to the intrinsic mechanical properties of the gut are easier to interpret from a physiological point of view.19 Furthermore, a test battery, where multimodal stimuli are used, will increase the probability for activation of a range of relevant nervous mechanisms in specific diseases.15 Recently, we developed a model where mechanical and thermal stimuli were combined,14,20 providing stimulation of both deeper (mechanical stimulation) and superficial (thermal stimulation) layers of the gut.21 We hypothesised that a multimodal stimulation approach would be able to elucidate differential pain mechanisms in patients with oesophagitis.
In patients with grade B oesophagitis and a matched control group the aims of the study were to investigate: (1) sensitivity to stimulation of the distal oesophagus using standardised mechanical stimulation before and after pharmacological relaxation of smooth muscle; (2) sensitivity to thermal stimuli with cold and heat in the distal oesophagus; and (3) referred pain areas to mechanical stimulation reflecting central pain mechanisms.
Material and methods
Selection of patients and controls
Eleven patients (nine men and two women) with grade B oesophagitis according to the Los Angeles classification22 were included in the study. All patients were recruited from the outpatient clinic at the Departments of Medical and Surgical Gastroenterology, Aalborg Hospital. Mean age was 49.7 (10.8) years. All had typical reflux symptoms with heartburn and/or acid regurgitation. GORD symptoms had lasted for more than three months in all patients but typically symptoms were intermittent for several years. All patients were treated with PPI until 48 hours before the study but they had not received surgical or endoscopic treatments. None received any other medication. Apart from reflux symptoms, they were healthy and had no disorders causing pain. Sixteen healthy volunteers, 13 men and three women (mean age 40.4 (11.8) years) served as controls. They were recruited from hospital and university staff. Volunteers were all healthy without pain complaints or signs of psychiatric disorders. In particular they denied having any heartburn or acid regurgitation, chest pain, dyspepsia, or irritable bowel‐like symptoms. All had normal physical examinations.
Participants gave written informed consent prior to participation, and the local ethics committee approved the protocol (No VN 2003/120 mch). Volunteers received compensation of DKK 400 (∼£40) and reimbursement of travelling expenses.
Mechanical stimulation
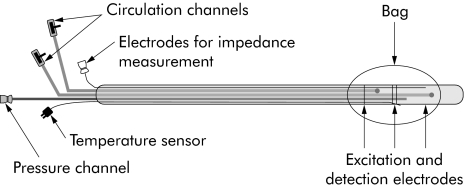
The probe used for stimulation is illustrated in fig 1. The impedance planimetric principle for measurement of cross sectional area (CSA) has been described in detail previously.19,23,24 The 70 cm long probe with a diameter of 4.5 mm had a cylindrical large sized bag near the tip. The bag was 40 mm in length and made of 35 μm thick non‐conducting polyestherurethane. It could be inflated to a CSA of approximately 2000 mm2 (diameter of 50 mm). The probe had a four electrode impedance planimetry system with four sets of ring electrodes inside the bag (GMC Aps, Hornslet, Denmark). The bag could be inflated with electrically conducting fluid (0.09% saline) through a pair of infusion channels, and the change in impedance of the fluid reflects the change in CSA.19 The infusion channels were connected to an infusion pump (type 111; Ole Dich Instrumentation, Hvidovre, Denmark) which could fill or empty the bag continuously. Fluid in the connecting tube between the pump and probe was heated to 37°C. A safety valve was connected to the pump allowing subjects to stop the infusion at any time. The system was calibrated before the probe was inserted into the oesophagus. Pressure was measured by means of a low compliance perfusion system connected to external transducers.
Figure 1 The probe used for mechanical and thermal stimulation of the distal oesophagus. The balloon was inflated with saline through the circulation channels. The impedance system consisted of excitation and detection electrodes inside the bag, allowing online measurement of the cross sectional area of the oesophagus. The circulation channels were also used for perfusion with cold and hot water. Temperature was monitored online with a temperature sensor, which ended inside the balloon. For details, see text.
Thermal stimulation
A 0.2 mm thick thermal electrode was placed inside a separate channel in the probe (Thermo‐element type K; Buhl and Bönsö A/S, Virum, Denmark) with the tip placed inside the balloon. The thermal electrode was connected to a transmitter (universal transmitter; PR Electronics, Rönde, Denmark) which again was connected to the impedance measuring system. Cold and heat pain stimuli were given with recirculation of 150 ml of water in the bag. The probe had two perfusion channels, and the channels were attached to a specially designed manual pump system where water was infused into one channel and simultaneously sucked out into the other channel at a speed of 100 ml/min.20,21 Syringes with water were kept in water baths at the desired temperatures. CSA was monitored during stimulation, and was kept constant during thermal stimuli.
Sensory assessment
Before the experiment, subjects were instructed on how to use the 0–10 electronic visual analogue scale (VAS) (GMC Aps, Hornslet, Denmark), where 0 = no perception; 1 = vague perception; 2 = definite perception of mild sensation; 3 = vague perception of moderate sensation; 4 = definite perception of moderate perception; 5 = pain threshold; 6 = mild pain; 7 = moderate pain; 8 = pain of medium intensity; 9 = intense pain; and 10 = unbearable pain. The scale has been described in detail previously.15 It is robust and discriminates sensations in the oesophagus with high accuracy.15 All subjects were able to tolerate distensions up to 7 on the scale but above this intensity widespread pain and autonomic reactions did not allow us to evoke higher pain intensities. Subjects were carefully instructed on how to score the evoked chest pain and to differentiate this from the unpleasantness in the throat caused by traction due to distension evoked oesophageal contractions.
During the stimuli, subjects were asked for any referred pain corresponding to the maximal pain evoked (VAS = 7). If present, the area of referred pain at the pain threshold was marked with a pen and transferred to a transparent paper and digitised (ACECAD D900+; Digitizer, Taiwan).
Protocol
Subjects fasted for at least four hours prior to the experiment. The probe was lubricated with moisturiser without local anaesthetics, and intubation was performed through the mouth. The bag was inserted into the stomach and then retracted to identify the location of the lower oesophageal sphincter as a zone of high resting pressure which decreased with swallowing. The bag was then placed 7 cm proximal to the sphincter, and the probe was taped to the chin. Subjects were asked to lie down with the head tilted at 30° after placement of the bag. The experiment was performed in this position after 30 minutes of rest. Patients and controls were not informed about the distension protocol and they were not able to see the monitor displaying the data. They were only informed about the start of the stimulus and that they had to score local pain intensity and referred pain area.
Preconditioning
Three bag distension stimuli with a constant infusion rate of 25 ml/min were carried out to precondition the tissue and to obtain repeatable sensory data.15 The interstimulus interval was 60 seconds for all experiments. When the subjects scored 5–6 on the VAS, the bag was deflated using the same flow rate as during inflation until it was empty.
Mechanical stimulation
After the preconditioning stimuli, two distensions were carried out at the same infusion rate until moderate pain intensity (7 on the VAS) was reached, after which the pump was reversed and the bag deflated by the same rate. Hereafter, 20 mg butylscopolamine were given intravenously to block distension induced peristalsis and to determine whether any differences between the groups could be attributed to the muscle component. After abolishment of contractile activity, the distensions were repeated.
Thermal stimulation
The intensities of the thermal stimuli (5°C and 60°C) were based on previous dose‐response experiments where these temperatures were capable of evoking pain in the oesophagus.20,21 Firstly, the bag was filled with the volume needed to reach a vague perception of a mild sensation (3 on the scale described above). This volume ensured sufficient contact with the mucosa. As CSA was kept constant at this level during perfusion, an increase in bag volume could not interfere with perception. Immediately after the initial filling of the bag, 150 ml water at the desired temperature were recirculated for 90 seconds or until mild pain was reached (6 on the scale described above). Immediately after the stimulus, the bag was emptied in five seconds.
During all stimuli, the electrocardiogram, pulse rate, and respiration were monitored and displayed onscreen using a Biopac MP100 system (Biopac Systems Inc., Santa Barbara, California, USA).
Data analysis
Mechanical stimulation
Circumferential wall tension was calculated according to the law of Laplace for cylindrical structures as
T = ΔPr
where T is the circumferential wall tension, r is the balloon radius, and ΔP is the transmural pressure. The geometry of the oesophagus during distension can be considered circular except at very low pressure levels.19 Therefore, the radius was determined as
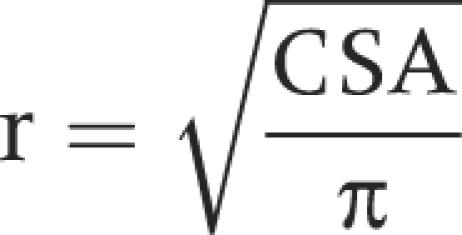 |
Visceral pain is diffuse and difficult to assess, especially when the referred pain area also needs to be quantified. To improve the psychophysical assessment of the pain intensity learning sessions are important.15 Thus after distensions to a VAS rating of 5–6 used for preconditioning the tissue, the first distension to 7 on the VAS was used as a test session. Subjects also stated that they rated the sensory intensity at the second distension to 7 on the VAS more reliably compared with the first. Therefore, only data from the second distension were used in the analysis. After butylscopolamine the first distension was used as the maximal decrease in contractile activity was seen during the first few minutes after injection.
Thermal stimulation
Initially there was a relatively slow change in temperature (mean 20 seconds) until the system was cooled/heated. After this, steady state was obtained where the temperature was relatively stable. However, time until the temperature was constant showed individual variation, and all subjects did not tolerate 90 seconds at the extreme temperatures. Thus the area under the temperature curve (AUC) was calculated in the range from 20 seconds after the start of the perfusions and for a duration of 90 seconds or until perfusion was stopped due to pain above 6 on the VAS scale. In this range the temperature inside the bag was constantly low/high, and the AUC was considered the best measure for the caloric load applied to the oesophageal wall.21
Statistics
Results are expressed as mean (SD) unless otherwise indicated. Continuous data were analysed using t tests. For multiple comparisons, two way analysis of variance was used with the factors: (1) patients versus controls and (2) different VAS levels. Test statistics for the F distribution (F) and probability values indicating statistical significance (p) are reported. A p value <0.05 was considered significant. The software package SigmaStat v. 3.0 was used for statistical analysis.
Results
Mechanical stimulation
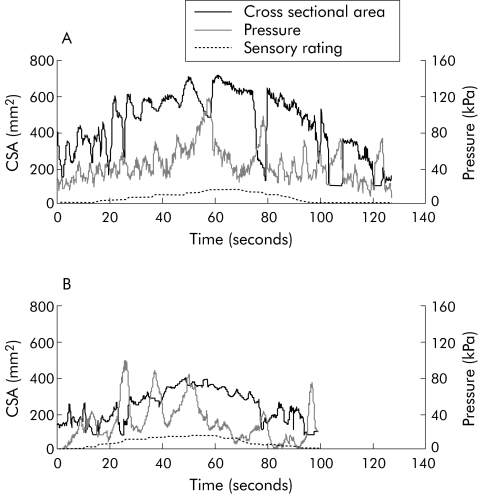
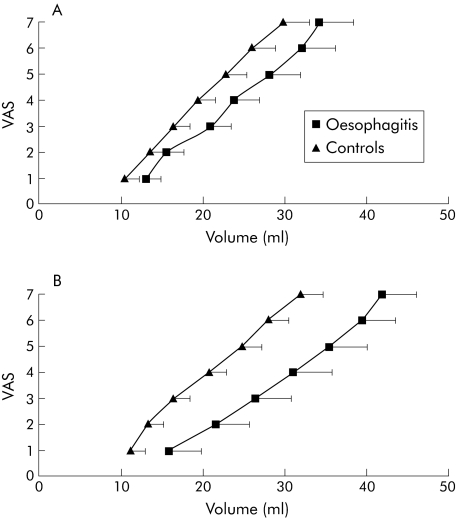
All subjects completed the experiment. Distensions resulted in a feeling of pressure and/or heartburn in both patients and controls but there were no differences in qualitative reporting. After the preconditioning stimuli, CSA and pressure curve characteristics and sensory ratings became reproducible in all subjects. In fig 2 an example of the volume and sensory rating is seen in a typical patient and a control subject. The number of contractions with pressure amplitudes above 2.5 kPa during the distensions was higher in patients compared with those evoked in controls (6.4 (2.9) and 3.2 (1.6); p = 0.001). Sensory responses to the mechanical stimulus after preconditioning are shown in fig 3 (stimulus‐response curves for volume). Patients were hyposensitive to mechanical stimuli assessed by volume (F = 8.1, p = 0.005). For CSA, pressure, and tension, graphs were nearly identical in the two groups (not shown) and there were no differences between the groups (CSA: F = 0.2, p = 0.6; pressure: F = 1.5, p = 0.2; tension: F = 2.1, p = 0.2). Lack of difference in CSA most likely reflects the fact that the contractile pattern in oesophagitis patients squeezed the bag which elongated, resulting in a relatively lower CSA despite increased volume.25
Figure 2 Raw data showing cross sectional area, pressure, and sensory rating (scale not shown) in a typical patient with oesophagitis (A) and a healthy control subject (B). The sensation was rated continuously on a visual analogue scale during distension of the oesophagus with a bag at 25 ml/min using an infusion pump. At moderate pain the pump was reversed and fluid withdrawn. The patient tolerated higher mechanical stimulus intensity compared with the control subject, as reflected in the higher cross sectional area and longer infusion time (higher volume).
Figure 3 Stimulus‐response curves. Sensory ratings on a visual analogue scale (VAS, with 5 as the pain threshold) in patients with oesophagitis and controls during distension of the oesophagus at 25 ml/min (A). Distensions were given until moderate pain was reported (7 on the VAS). In (B) the same data are shown after pharmacological relaxation of smooth muscle. Patients with oesophagitis were hyposensitive to the mechanical stimuli. Values are mean (SEM).
After relaxation of smooth muscle with butylscopolamine, the difference between the stimulus‐response curves for volume was more evident (F = 27.4, p<0.001) (fig 3) whereas the stimulus‐response curves for CSA, pressure, and tension did not differ between the groups (all p>0.2). Thus pharmacological relaxation of the distension evoked secondary contractions seemed to influence bag volume to a higher degree in oesophagitis patients characterised by increased secondary contractions at baseline.
Thermal stimulation
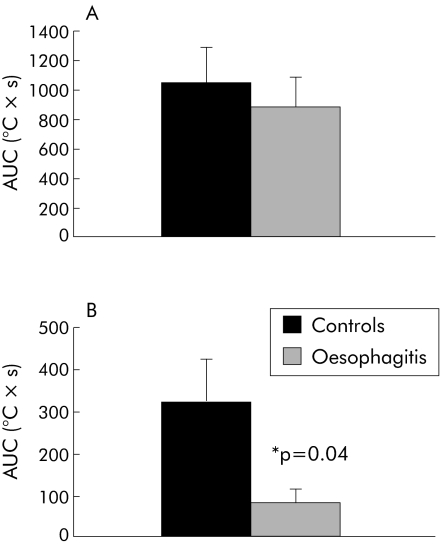
Stimulation at 37°C was not felt by any of the subjects, excluding a small component in the pain response due to the slight bag distension during thermal stimulation. The 5°C and 60°C stimuli were reported as cold and warm/burning, respectively, in the majority of both patients and controls. In fig 4, AUC values for cold and heat stimulation are shown in the two groups. The AUC value for cold stimulation was 1048.6 (242.7) °C×s in controls and 889.8 (202.6) °C×s in patients (p = 0.5). For the heat stimuli, AUC values were 323.3 (104.1) and 81.3 (32.3) °C×s in controls and patients (p = 0.04), respectively. As the AUC value was computed at stable temperatures, the difference was mainly due to shorter tolerated stimulation time in the oesophagitis group.
Figure 4 Caloric load determined as the area under the curve (AUC) for cold (A) and heat (B) pain stimuli of the oesophagus in patients with oesophagitis and healthy controls. To improve the details, scales on the y axis are not the same. There was a significant difference between the groups for heat pain stimuli. Values are mean (SEM).
Referred pain areas
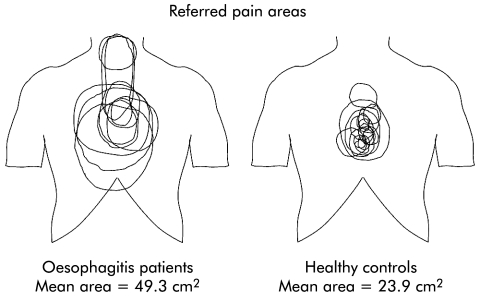
As shown in fig 5, all subjects reported referred pain to mechanical stimulation. CSA of the oesophagus was similar in the two groups at moderate pain intensity (562.7 mm2 in controls and 546.1 mm2 in patients; p = 0.9). For controls, mean referred pain area was 23.9 (7) cm2 and for patients mean area was 49.3 (6.2) cm2 (p = 0.02). Referred pain was widely distributed in patients whereas it was more centred in the chest in healthy controls.
Figure 5 Referred pain area to mechanical stimulation of the oesophagus in patients with oesophagitis and in controls. The figure was constructed by drawings made by the subjects at moderate pain intensity (7 on the visual analogue scale). Additionally, three patients had spread to the neck, and one patient and two controls had spread of the referred pain to the back (data not shown). The referred pain area was larger and more widespread in patients.
Discussion
The study is the first to compare the pain response with multimodal stimuli of the oesophagus in patients with oesophagitis and in controls. Patients with grade B oesophagitis had hyposensitivity to the infused volume of the bag and showed an increased number of distension induced contractions but had hyperalgesia to heat and increased/widespread referred pain to mechanical stimuli. We believe that acid reflux in vivo specifically sensitises heat receptors in the mucosa and evokes central changes reflected in the referred pain pattern. These observations are consistent with experimental studies using short lasting acid perfusion of the distal oesophagus in healthy volunteers, and may be important in our understanding of the pain mechanisms in patients with erosive reflux disease.
Acid related changes in the sensation of oesophageal pain
Mechanical stimulation
In an animal study, Garrison and colleagues12 demonstrated that spinal neurones in the cat receiving input from the distal oesophagus also received convergent input from the thoracic wall. When the oesophagus was sensitised with turpentine, neurones responded to a smaller mechanical stimulus. Compared with experiments in animals, it is not possible to give an exact definition of the neurophysiological changes observed in the nervous system following sensitisation in humans. What we observe in the oesophagus is most likely a combination of peripheral and central sensitisation. In humans, relatively few studies have been done to explore the pain mechanisms in GORD. Trimble and colleagues26 showed that patients with non‐erosive reflux disease (NERD) and normal ambulatory pH monitoring had increased sensation to distension of the oesophagus whereas the sensation threshold in those with excess reflux was normal. Rodriguez‐Stanley and colleagues16 also reported a decrease in sensation to distension in NERD patients compared with an historic control group. Although the methods were different from those in the current study, the finding reflects the fact that patients with pathological acid reflux may be less sensitive to mechanical stimulation. These results are similar to those in the current study. Patients with erosive oesophagitis constitute less than 20% of patients,27 and it is questionable whether GORD is a spectrum of disease or whether patients with erosive disease constitute a unique group of patients.3 In a study comparing patients with oesophagitis with controls, Fass and colleagues28 demonstrated enhanced perception to acid perfusion but the stimulus‐response functions and location of the referred pain area to phasic and slow‐ramp distensions were normal. Such findings may point towards a differential effect on mechanosensitive and chemosensitive pathways in oesophagitis. In contrast with this study, we found hypoalgesia to mechanical stimulation, as assessed by the infused volume, an effect which was more pronounced after pharmacological relaxation of smooth muscle. Methodological considerations may partly explain these differences, and in the latter study the referred pain area was not quantified. We cannot exclude the fact that lack of evidence for sensitisation to mechanical stimuli may be related to our distension protocol. Hence in the human rectum, phasic distensions were shown preferentially to stimulate spinal pathways thought to mediate pain whereas slow tonic stimuli mainly affect parasympathetic nerves.29 The same could be the case for innervation of the oesophagus. On the other hand, the slow ramp distension used in the current study was more physiological, and time dependent viscoelastic effects are avoided after preconditioning of the tissues.15,30 Furthermore, after relaxation of smooth muscle with butylscopolamine, we were able to decrease the number and amplitude of the secondary contractions and showed that hypoalgesia to bag volume was related more to passive tissue behaviour. We do not believe that butylscopolamine had any effects on the sensory pathways as cholinergic blockade in the oesophagus does not change the sensation to electrical stimuli or mechanical stimuli of the oesophagus.31,32
Normally, strain or CSA is considered the most reliable parameter for predicting the sensory response to distension of the oesophagus32 but in the current study there were no differences between the two groups for this parameter. This can be explained most likely by the high frequency of the secondary contractions in oesophagitis patients which may squeeze the bag resulting in elongation and hence relatively low CSA despite higher volumes. Evidence for such squeezing was recently demonstrated in healthy subjects exposed to acid perfusion.25 Furthermore, if we had plotted the data as a function of CSA we would have encountered the issue of active versus passive tissue properties, a topic which is not necessarily easily accounted for.25 Hence the contractile pattern thought to be evoked by the acid makes it very difficult to determine the adequate mechanical stimulus.
Hypoalgesia to experimental visceral stimulation was also seen in patients with other chronic visceral diseases, such as Crohn's disease33 and peptic ulcer.34 This is contrasted by the pain in functional visceral disorders such as irritable bowel syndrome and non‐ulcer dyspepsia where hyperalgesia and allodynia to experimental stimuli of the gut are typically found.33,34,35,36,37 Such findings suggest that counter‐regulatory mechanisms may prevent the mechanical hyperalgesia in organic diseases.28 It can be speculated that a change in the balance between noxious control systems arising in the brainstem may explain the findings. The central pain modulating systems rely on a balance between facilitatory and inhibitory descending pathways and intrinsic spinal circuits.38,39 Studies on animals have shown that the system is an important mechanism in the modulation of visceral stimuli,40 and descending inhibitory control is probably also important in modulating chronic visceral pain in humans.
Thermal stimulation
Patients showed hyperalgesia to heat (but not cold) stimuli. As thermal stimulation was induced at a bag volume corresponding to 3 on the VAS (vague perception of moderate sensation), different effects of costimulation of mechano and heat sensitive pathways could theoretically be important. However, as patients were hyposensitive to mechanical stimulation this would counteract the hypersensitivity to heat stimuli, and therefore the mechanical stimulus used to unfold of the bag probably did not interfere with the results. Heat specific hyperalgesia is consistent with findings in experimental pain studies in our laboratory. Recently, we showed that acid perfusion of the oesophagus in healthy subjects differentially sensitises the oesophagus to heat but not cold stimuli.21 Animal experiments have shown that thermal receptors exist throughout the gastrointestinal tract, including the oesophagus.8,41,42 The TRPV1 receptor may be important to our findings as it is activated as a polymodal detector of potential harmful stimuli, including noxious heat and protons.43,44 Capsaicin also activates the receptor, and we have recently shown that capsaicin applied to the ileum evoked visceral and referred somatic pain, together with visceral hyperalgesia.45 TRPV1 receptors have previously been demonstrated in the human oesophagus, and the receptor is upregulated in oesophagitis.46 Furthermore, in the oesophagus, application of capsaicin sensitised the oesophagus to acid reflux.47 Although we did not measure the amount of acid reflux, excess acid exposure can be detected in more than 90% of patients with erosive disease.48 Thus in patients with oesophagitis, acid reflux and resulting peripheral sensitisation probably results in a significant change in the sensation to heat stimuli via TRPV1 receptors.
Central changes
Acid reflux in patients with oesophagitis may result in central sensitisation. In healthy subjects we have previously shown that acid perfusion of the distal oesophagus resulted in an increase in the amplitude of the polysynaptic withdrawal reflex, and in a larger referred pain area to differentiated oesophageal stimuli, reflecting central hyperexcitability.14 Furthermore, Sarkar and colleagues13,49 recently demonstrated that acid perfusion of the distal oesophagus resulted in allodynia and shorter latencies of the evoked brain potentials to electrical stimuli of a more proximal segment of the oesophagus not exposed to the acid (secondary visceral hyperalgesia). In the current study, the larger and more widespread localisation of the referred pain area in patients is thought to represent such central neuroplastic changes. This is most likely related to hyperexcitability and subsequent opening of latent connections between converging neurones from visceral and somatic structures in the CNS.50 Penagini and colleagues51 showed that patients with oesophagitis had increased sensitivity to distension of the proximal stomach. This may be a result of visceral nerves from the stomach and oesophagus converging on hyperexcitable neurones in the CNS. This viscero‐visceral convergence may reflect the same mechanisms which are those explaining the increased referred pain area. Thus there is substantial evidence that exposition of acid in the oesophagus in patients with oesophagitis results in central neuroplastic changes. The balance between central hyperexcitability and descending inhibitory control is not predictable. These neuroplastic changes may result in increased referred pain on the one hand and dampening of the activity from mechanosensitive pathways on the other. The mechanisms controlling this balance are not understood and current knowledge does explain the discrepancy.
Nearly half of patients with oesophagitis continue to complain of heartburn and regurgitation even after one month of treatment (where the erosive changes have disappeared in most patients), and not all patients have complete relief of symptoms despite adequate and longlasting treatment.52 As central hyperexcitability and neuroplastic changes to visceral diseases often persist even after the original disease has disappeared,11 the central changes demonstrated in the current study may help explain the symptoms in the subgroup of patients which continue to complain of reflux‐like symptoms despite adequate therapy. In this subgroup, it may be useful to try pharmacological interventions targeting central pain mechanisms.
Conclusion
The data indicate that peripheral sensitisation of heat sensitive pathways combined with facilitation of central pain mechanisms may contribute to our understanding of the symptoms in patients with oesophagitis. This has important implications for the classification, clinical understanding, and treatment of patients with erosive reflux disease.
Acknowledgements
The study was supported from “Nordjyllands Amts Forskningslegat”, “Det Obelske Familiefond”, and “Spar Nord Fonden”.
Abbreviations
AUC - area under the temperature curve
CNS - central nervous system
CSA - cross sectional area
GORD - gastro‐oesophageal reflux disease
NERD - non‐erosive reflux disease
PPI - proton pump inhibitors
VAS - visual analogue scale
Footnotes
Conflict of interest: None declared.
References
- 1.Mahmood Z, McNamara D. Gastro‐oesophageal reflux disease and ulcer disease. Aliment Pharmacol Ther 200318(suppl 3)31–37. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson R, Frison L, Lundell L.et al Relationship between symptoms, endoscopic findings and treatment outcome in reflux esophagitis. Gastroenterol 1996110A77 [Google Scholar]
- 3.Fass R, Tougas G. Functional heartburn: the stimulus, the pain, and the brain. Gut 200251885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto K, Iwakiri R, Mori M.et al Clinical symptoms in endoscopic reflux esophagitis: evaluation in 8031 adult subjects. Dig Dis Sci 2003482237–2241. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson R, Dent J, Bolling‐Sternevald E.et al The usefulness of a structured questionnaire in the assessment of symptomatic gastroesophageal reflux disease. Scand J Gastroenterol 1998331023–1029. [DOI] [PubMed] [Google Scholar]
- 6.Bytzer P. Goals of therapy and guidelines for treatment success in symptomatic gastroesophageal reflux disease patients. Am J Gastroenterol 200398S31–S39. [DOI] [PubMed] [Google Scholar]
- 7.Milkes D, Gerson L B, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease (GERD). Am J Gastroenterol 200499991–996. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta J N, Gebhart G F. Gastrointestinal afferent fibers and sensation. In: Johnson L, ed. Physiology of the gastrointestinal tract. New York: Raven Press, 1994484–519.
- 9.Cervero F. Sensory innervation of the viscera: Peripheral basis of visceral pain. Physiol Rev 19947495–138. [DOI] [PubMed] [Google Scholar]
- 10.DeVault K R, Beacham S, Castell D O.et al Esophageal sensation in spinal cord‐injured patients: Balloon distension and cerebral evoked potential recording. Am J Physiol Gastrointest Liver Physiol 199634G937–G941. [DOI] [PubMed] [Google Scholar]
- 11.Giamberardino M A. Recent and forgotten aspects of visceral pain. Eur J Pain 1999377–92. [DOI] [PubMed] [Google Scholar]
- 12.Garrison D W, Chandler M J, Foreman R D. Viscerosomatic convergence onto feline spinal neurons from esophagus, heart and somatic fields—effects of inflammation. Pain 199249373–382. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar S, Aziz Q, Woolf C J.et al Contribution of central sensitisation to the development of non‐cardiac chest pain. Lancet 20003561154–1159. [DOI] [PubMed] [Google Scholar]
- 14.Drewes A M, Schipper K ‐ P, Dimcevski G.et al Multi‐modal induction and assessment of allodynia and hyperalgesia in the human oesophagus. Eur J Pain 20037539–549. [DOI] [PubMed] [Google Scholar]
- 15.Drewes A M, Gregersen H, Arendt‐Nielsen L. Experimental pain in gastroenterology: A reappraisal of human studies. Scand J Gastroenterol 2003381115–1130. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez‐Stanley S, Robinson M, Earnest D L.et al Esophageal hypersensitivity may be a major cause of heartburn. Am J Gastroenterol 199994628–631. [DOI] [PubMed] [Google Scholar]
- 17.Trimble K C, Farouk R, Pryde A.et al Heightened visceral sensation in functional gastrointestinal disease is not site‐specific. Evidence for a generalized disorder of gut sensitivity. Dig Dis Sci 1995401607–1613. [DOI] [PubMed] [Google Scholar]
- 18.Fass R, Fennerty M B, Ofman J J.et al The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterol 199811542–49. [DOI] [PubMed] [Google Scholar]
- 19.Gregersen H.Biomechanics of the gastrointestinal tract. London: Springer Verlag, 2002
- 20.Drewes A M, Schipper K P, Dimcevski G.et al Multimodal assessment of pain in the esophagus: a new experimental model. Am J Physiol Gastrointest Liver Physiol 2002283G95–103. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen J, Reddy H, Funch‐Jensen P.et al Cold and heat pain assessment of the human oesophagus after experimental sensitisation with acid. Pain 2004110393–399. [DOI] [PubMed] [Google Scholar]
- 22.Lundell L R, Dent J, Bennett J R.et al Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 199945172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregersen H, Andersen M B. Impedance measuring system for cross‐sectional area in the gastrointestinal tract. Med Biol Eng Comput 199129108–110. [DOI] [PubMed] [Google Scholar]
- 24.Gregersen H, Giversen I M, Rasmussen L M.et al Biomechanical wall properties and collagen content in the partially obstructed opossum esophagus. Gastroenterology 19921031547–1551. [DOI] [PubMed] [Google Scholar]
- 25.Drewes A M, Reddy H, Staahl C.et al Sensory‐motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol 2005114367–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trimble K C, Pryde A, Heading R C. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro‐oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut 1995377–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatlebakk J G, Hyggen A, Madsen P H.et al Heartburn treatment in primary care: randomised, double blind study for 8 weeks. BMJ 1999319550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fass R, Naliboff B, Higa L.et al Differential effect of long‐term esophageal acid exposure on mechanosensitivity and chemosensitivity in humans. Gastroenterol 19981151363–1373. [DOI] [PubMed] [Google Scholar]
- 29.Lembo T, Munakata J, Mertz H.et al Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterol 19941071686–1696. [DOI] [PubMed] [Google Scholar]
- 30.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil 19968277–297. [DOI] [PubMed] [Google Scholar]
- 31.Barlow J D, Gregersen H, Thompson D G. Identification of biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol Gastrointest Liver Physiol 2002282G683–G689. [DOI] [PubMed] [Google Scholar]
- 32.Drewes A M, Pedersen J, Liu W.et al Controlled mechanical distension of the human oesophagus: Sensory and biomechanical findings. Scand J Gastroenterol 20033827–35. [PubMed] [Google Scholar]
- 33.Bernstein C N, Niazi N, Robert M.et al Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain 199666151–161. [DOI] [PubMed] [Google Scholar]
- 34.Mertz H, Fullerton S, Naliboff B.et al Symptoms and visceral perception in severe functional and organic dyspepsia. Gut 199842814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munakata J, Naliboff B, Harraf F.et al Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome (published erratum appears in Gastroenterology 1997;113:1054). Gastroenterology 199711255–63. [DOI] [PubMed] [Google Scholar]
- 36.Rössel P, Drewes A M, Petersen P.et al Pain produced by electric stimulation of the rectum in patients with irritable bowel syndrome: further evidence of visceral hyperalgesia. Scand J Gastroenterol 1999341001–1006. [DOI] [PubMed] [Google Scholar]
- 37.Schmulson M, Chang L, Naliboff B.et al Correlation of symptom criteria with perception thresholds during rectosigmoid distension in irritable bowel syndrome patients. Am J Gastroenterol 200095152–156. [DOI] [PubMed] [Google Scholar]
- 38.Lebars D, Dickenson A H, Besson J M. Diffuse noxious inhibitory controls (Dnic).1. Effects on dorsal horn convergent neurons in the rat. Pain 19796283–304. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki R, Morcuende S, Webber M.et al What the brain tells the spinal cord: Lamina I/III NK1‐expressing neurons control spinal activity via descending pathways. In: Dostrovsky JO, Carr DB, Koltzenburg M, eds. Proceedings of the 10th World Congress on Pain, progress in pain research and management. Seattle: IASP Press, 2003337–344.
- 40.Roza C, Laird J M, Cervero F. Spinal mechanisms underlying persistent pain and referred hyperalgesia in rats with an experimental ureteric stone. J Neurophysiol 1998791603–1612. [DOI] [PubMed] [Google Scholar]
- 41.Ouazzani T E, Mei N. Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 198283995–1001. [PubMed] [Google Scholar]
- 42.Fall M, Lindström S, Mazières L. A bladder‐to‐bladder cooling reflex in the cat. J Physiol 1990427281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maggi C A. The dual, sensory and “efferent” function of the capsaicin‐sensitive primary sensory neurons in the urinary bladder and uretra. In: Maggi CA, ed. Nervous control of the urogenital system. Chur: Harwood Academic Publishers, 1993382–422.
- 44.Caterina M J. Vanilloid receptors take a TRP beyond the sensory afferent. Pain 20031055–9. [DOI] [PubMed] [Google Scholar]
- 45.Drewes A M, Schipper K ‐ P, Dimcevski G.et al Gut pain and hyperalgesia induced by capsaicin: A human experimental model. Pain 2003104333–341. [DOI] [PubMed] [Google Scholar]
- 46.Matthews P J, Aziz Q, Facer P.et al Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol 200416897–902. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez R, Dunkel R, Koletzko B.et al Effect of capsaicin‐containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig Dis Sci 1998431165–1171. [DOI] [PubMed] [Google Scholar]
- 48.Kahrilas P J, Dodds W J, Hogan W J.et al Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology 198691897–904. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar S, Hobson A R, Furlong P L.et al Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 2001281G1196–G1202. [DOI] [PubMed] [Google Scholar]
- 50.Arendt‐Nielsen L, Laursen R J, Drewes A M. Referred pain as an indicator for neural plasticity. Prog Brain Res 2000129343–356. [DOI] [PubMed] [Google Scholar]
- 51.Penagini R, Hebbard G, Horowitz M.et al Motor function of the proximal stomach and visceral perception in gastro‐oesophageal reflux disease. Gut 199842251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talley N J. Review article: gastro‐oesophageal reflux disease—how wide is its span? Aliment Pharmacol Ther 200420(suppl 5)27–37. [DOI] [PubMed] [Google Scholar]