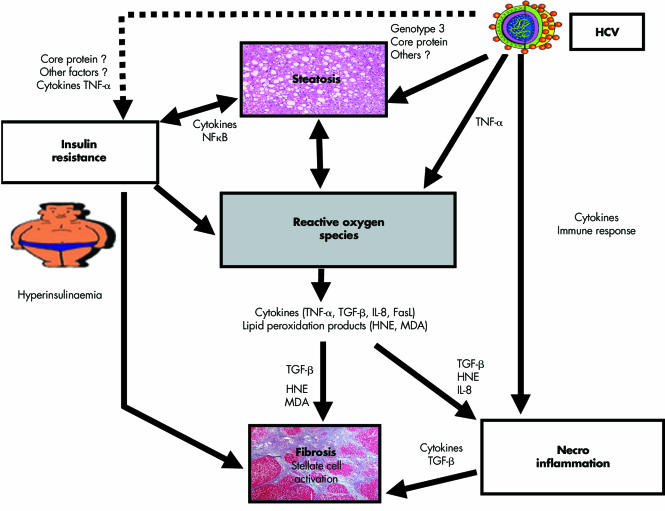
Figure 2 Pathogenesis of liver fibrosis in chronic hepatitis C. Proposed mechanisms leading to steatosis and fibrosis in hepatitis C virus (HCV) infection. Steatosis may be caused directly by HCV core protein expression (with or without the cooperation of other viral proteins) but other mechanisms are likely, such as the hyperglycaemic/hyperinsulinaemic state, the hallmark of insulin resistance (where HCV may play an additional direct role through activation of cytokines), and production of reactive oxygen species (ROS) (again, possibly induced by HCV and by insulin resistance). Furthermore, steatosis renders hepatocytes more insulin resistant and, on the other hand, provides the fuel for amplifying the production of ROS. Fatty accumulation, however, is not fibrogenic per se. The production of ROS may provide a pathogenetic link between steatosis and fibrosis. ROS induce cytokines formation by hepatocytes (tumour necrosis factor α (TNF‐α), transforming growth factor β (TGF‐β), interleukin 8 (IL‐8), and FasLigand (FasL)). TGF‐β activates collagen synthesis by stellate cells while IL‐8 is a potent chemoattractant for human neutrophils. ROS trigger lipid peroxidation, which releases malondialdehyde (MDA) and 4‐hydroxynonenal (HNE). MDA and HNE also increase collagen synthesis by stellate cells while HNE is a potent chemoattractant for human neutrophils. Additional fibrogenetic stimuli are represented by the fibrogenic inflammatory cytokines (released as part of both innate and adaptive immune responses to HCV infection and antigen expression) and by the direct profibrogenic effect of hyperinsulinaemia.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.