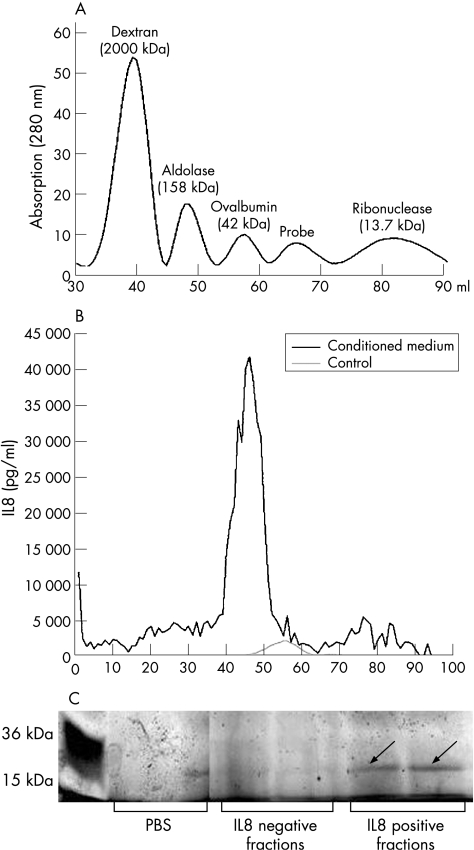
Figure 6 (A) Standard proteins of known molecular size were separated by fast performance liquid chromatography (FPLC) to determine the elution volume of the HiPrep 16/60 Sephacryl S‐200 High Resolution column. The chromatogram was used to determine the molecular size of the unknown protein in colonic epithelial cells (CEC)‐conditioned media. (B) FPLC was used to further purify the colonic lamina propia fibroblasts (CLPF)‐stimulating factor from CEC‐conditioned medium. A volume of 50 ml of CEC‐conditioned media containing CEC isolated from each of 11 surgical specimens was collected, the protein was precipitated, and the protein mix supplied to the column. Total elution volume was 120 ml; the first 20 ml were discarded. Each fraction was tested for its interleukin 8 (IL8)‐inducing effect on CLPFs. In control (unconditioned) media, no fraction significantly induced IL8 secretion. In conditioned media, fractions 42–52 induced strong IL8 secretion. This area was used for further analysis. (C) The fractions of FPLC chromatography (IL8‐inducing fractions, non‐IL8‐inducing fractions, and phosphate‐buffered saline (PBS) as control; See BluePlus 2 as standard) were used for protein gel analysis. A mixture of bands of different molecular sizes or no bands in the IL‐8‐negative fractions was found. In the IL8‐positive fractions, there was one clear band (arrow) at a molecular size of about 30 kDa that did not appear in the other lanes. This area was separated and analysed by matrix‐assisted laser desorption/ionisation time‐of‐flight analysis.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.