Abstract
Background
Triple therapy is recommended for Helicobacter pylori eradication, yet consensus on the duration of treatment is lacking.
Aim
To compare the efficacy and safety of 1‐ and 2‐week regimens of omeprazole, amoxicillin and clarithromycin in a large, multicentre, double‐blind and randomised study.
Methods
A total of 909 H pylori‐positive patients with duodenal ulcer, enrolled in 81 endoscopy units in Italy, were randomised to receive omeprazole, amoxicillin and clarithromycin for either 1 week (OAC1W) or 2 weeks (OAC2W) or omeprazole and amoxicillin for 2 weeks. H pylori eradication was assessed by histological examination and carbon‐13 urea breath test 4 weeks after treatment.
Results
Both the intention‐to‐treat (ITT; n = 907) and per protocol (PP; n = 661) analyses showed no significant differences between the eradication rates of OAC1W (ITT 79.7%; PP 83.6%) and OAC2W (ITT 81.7%; PP 84.9%; ITT p = 0.53; PP p = 0.71). Both triple omeprazole, amoxicillin and clarithromycin regimens gave significantly higher eradication rates compared with omeprazole and amoxicillin treatment (ITT 44.6%; PP 42.8%; p<0.001). Poor compliance was reported in 18.6%, 17.3% and 15.1% (p = 0.51) of patients for OAC2W, OAC1W and omeprazole and amoxicillin, respectively. Adverse events occurred in 9.9% and 9.6% (p = 0.88) of patients for OAC2W and OAC1W, respectively, and in 5.9% for omeprazole and amoxicillin (p = 0.11).
Conclusions
1‐week and 2‐week triple treatments for H pylori eradication are similar in terms of efficacy, safety and patient compliance.
Helicobacter pylori infection is now recognised as a major cause of peptic ulcer disease, and several studies have shown that its eradication markedly reduces the rate of ulcer relapse.1,2 Multicentre studies have shown that triple therapy with omeprazole, clarithromycin and either amoxicillin or metronidazole is one of the most effective and well‐tolerated treatments for H pylori eradication.3,4,5,6 However, a consensus on the length of treatment is still lacking. In Europe, guidelines for treatment of H pylori infection recommend 1 week of treatment,7 whereas in the USA it is recommended that triple regimens be given for 10–14 days.8,9 In recent years, a decrease in the efficacy of 1 week of triple therapy has been reported.10,11,12 A longer duration of treatment could provide better eradication rates. Some large, multicentre studies have recently reported that prolonging the duration of triple therapy from 7 to 10 days does not improve its results.12,13,14 Individual studies comparing treatment with a proton pump inhibitor (PPI) and two antibiotics for 1 and 2 weeks have generally shown little difference between the two regimens.11,15,16,17,18,19,20,21 However, two meta‐analyses have concluded that 2‐week triple therapies achieve considerably better results than 1‐week schedules.22,23 Overall, few studies have directly compared 1‐ and 2‐week triple regimens and the sample sizes of those that have been performed are not sufficiently large to detect any noticeable difference in efficacy.11,15,16,17,18,19,21
To overcome this shortcoming, we performed a large, randomised, multicentre trial involving >900 consecutive patients with duodenal ulcer enrolled in 81 Italian endoscopy units. The aim of this double blind, placebo‐controlled study was to compare the efficacy and safety of 1 week and 2 weeks of omeprazole‐based triple therapy, including amoxicillin and clarithromycin, in H pylori eradication in patients with duodenal ulcer. A standard dual therapy (omeprazole and amoxicillin) was included as a control arm with a low‐eradication rate. This was included both as a measure of the internal validity of the study, and as part of a second long‐term follow‐up study which had the aim of evaluating factors associated with the recurrence of duodenal ulcer, such as sex, age, smoking, positive family history for peptic ulcer, and dyspeptic and reflux symptoms.
Materials and methods
Patients
Patients with a symptomatic duodenal ulcer who were H pylori‐positive according to rapid urease testing were enrolled in the study between May 1996 and June 1998. Patients with a prepyloric ulcer within 2 cm of the pylorus were also accepted. The following exclusion criteria were applied: previous treatments for H pylori eradication; allergy to penicillin or macrolides; significant liver or kidney disease; severe cardiac or pulmonary disease; suspected or confirmed malignancy; concurrent gastric ulcer or reflux oesophagitis; active upper gastrointestinal bleeding; history of gastric surgery; pregnancy or breast feeding; and clinically significant abnormalities in the predrug laboratory screen. Patients using antibiotics in the month before inclusion, bismuth‐containing compounds during the 3 months before inclusion, or PPIs, H2‐receptor antagonists, misoprostol or sucralfate in the 2 weeks before the pre‐entry endoscopy were excluded. Patients receiving regular treatment with non‐steroidal anti‐inflammatory drugs (⩾5 days a week, for at least 2 weeks during the month before the start of the study) were also excluded.
This study was performed according to good clinical practice and the Declaration of Helsinki, and was approved by the research ethics committee of each participating centre. Written informed consent was obtained from all patients.
Study design
This study had a double blind, randomised, placebo‐controlled, parallel‐group design and involved 81 Italian endoscopy units. Patients were randomised using a computerised random‐number generator to receive omeprazole 20 mg twice daily in combination with amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 (OAC1W) or 2 weeks (OAC2W), or omeprazole 20 mg twice daily, amoxicillin 1 g twice daily and clarithromycin placebo twice daily for 2 weeks (hereafter referred to as omeprazole and amoxicillin). Patients in the OAC1W group were treated for one additional week with omeprazole 20 mg twice daily and amoxicillin and clarithromycin placebos twice daily. Treatments were assigned to patients using numbered containers and the randomisation was centralised and implemented by AstraZeneca, Italy. The computer‐generated randomisation list was blocked by centre into blocks of size 2; the block size was unknown to the investigators. Patient numbers were allocated consecutively at the different centres at the time of enrolment.
Endoscopy was performed at the end of the 2‐week administration period for the evaluation of ulcer healing, which was defined by the presence of white scars or complete re‐epithelisation in the area where the ulcer was present earlier. In patients without healed ulcers, endoscopy was performed again after 4 weeks of treatment with omeprazole 20 mg twice daily. At 2 weeks, adverse events and treatment compliance were evaluated by personal interview. Treatment compliance was also measured by counting the returned pills. Patients were considered to be non‐compliant if <75% of study medication was taken.
Assessment of H pylori status
Biopsy specimens were taken during upper gastrointestinal endoscopy before the start of the study treatment. Two biopsy specimens, one from the corpus and one from the antrum, were taken for the rapid urease test (CP test, Yamanouchi Pharma SpA, Carugate, Milan, Italy). For histological assessment of H pylori infection, two biopsy specimens were taken from the corpus and three from the antrum. H pylori infection was also assessed by carbon‐13 urea breath test (13C‐UBT). Although all patients with a positive result for the rapid urease test were enrolled, patients were considered H pylori‐positive only if a positive result was confirmed by histological examination or 13C‐UBT. At least 4 weeks after the withdrawal of treatment, all patients with healed ulcers underwent endoscopy, where five biopsy specimens, two from the corpus and three from the antrum, were taken for histological examination. 13C‐UBT was also performed. Patients were considered H pylori‐negative if both the histological assessment and the 13C‐UBT gave negative results.
For H pylori diagnosis, biopsy specimens were fixed in 10% formalin and stained with haematoxylin–eosin and Giemsa stain. The 13C‐UBT was performed using 100 mg of 13C‐urea and a single post‐urea breath sample at 30 min according to the European standard protocol.24
Statistical analysis
Statistical analysis was performed using the SAS statistical package (V.6.12). The primary efficacy variable was the eradication rate of H pylori. Assuming true eradication rates of 85% and 94% for 1 and 2 weeks respectively for the OAC regimen, 203 patients in each group would be necessary to detect, with 80% power, a minimum difference of 9% between the two groups. This sample size was calculated using the normal approximation to the binomial distribution at a significance level of 0.05. Allowing for a 20% dropout rate, at least 254 patients for each group were required to be enrolled.
Intention‐to‐treat (ITT) and per protocol (PP) statistical analyses were performed. ITT analysis included all randomised patients who had taken at least one dose of study medication, except those in whom positive H pylori infection status at entry was not confirmed by either histological examination or 13C‐UBT. PP analysis included all patients who took at least 75% of the study medication, except those who were lost to follow‐up and those with major protocol violations that could have influenced treatment outcome. For example, patients were excluded if they used disallowed drug during the study, if the assessment of post‐treatment H pylori status was performed too early, if their H pylori status was unknown due to missing data, or if they had concurrent gastric ulcer or related diseases. The treatment groups were compared using the normal approximation to the binomial distribution. A χ2 test or Fisher's exact test, as appropriate, was performed to compare demographics characteristics and eradication rates between treatment groups, and the analyses were prespecified in the trial protocol. A p value of <0.05 was considered to be significant.
Results
A total of 909 patients (621 males, 288 females; mean (range) age, 46 (18–72) years) were randomised in this study: 302 patients in the OAC1W group, 302 in the OAC2W group and 305 in the omeprazole and amoxicillin group. No differences were observed among the three groups in terms of sex, age, smoking and alcohol use as shown in table 1.
Table 1 Baseline characteristics of study patients.
| OAC1W (n = 301) | OAC2W (n = 302) | OA (n = 305) | p Value | |
|---|---|---|---|---|
| Sex (male) | 206 (68.4%) | 216 (71.5%) | 198 (64.9%) | 0.19 |
| Age (years; mean (SD)) | 45.5 (11.8) | 46.7 (11.6) | 45.5 (11.5) | — |
| Smoking | ||||
| Yes | 172 (57.1%) | 166 (55.0%) | 151 (49.5%) | 0.15 |
| No | 85 (28.2%) | 94 (31.1%) | 102 (33.4%) | 0.38 |
| Ex‐smoker | 44 (14.6%) | 42 (13.9%) | 52 (17.0%) | 0.53 |
| Alcohol use | 133 (44.2%) | 153 (50.7%) | 139 (45.6%) | 0.24 |
OA, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and placebo for 2 weeks; OAC1W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 week, followed by omeprazole 20 mg twice daily and placebo for 1 week; OAC2W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 g twice daily for 2 weeks.
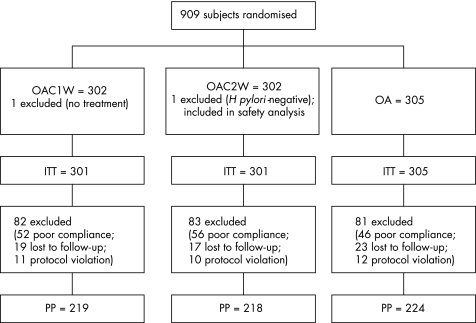
Two patients were excluded from the ITT analysis: one patient from the OAC1W group, who received no treatment, and one patient from the OAC2W group who was H pylori‐negative at entry. This last patient was only included in the safety analysis. A total of 11 patients from the OAC1W group, 10 patients from the OAC2W group and 12 patients from the omeprazole and amoxicillin group were excluded from the PP analysis due to major protocol violations. In all, 19 patients from the OAC1W group, 17 patients from the OAC2W group and 23 patients from the omeprazole and amoxicillin group were lost to follow‐up. Poor compliance also lead to exclusion from the PP analysis for a further 52 (17.3%) patients from the OAC1W group, 56 (18.6%) patients from the OAC2W group and 46 (15.1%) patients from the omeprazole and amoxicillin group. Treatment compliance was similar in the three groups (p = 0.51). Therefore the PP analysis of H pylori eradication included 219 patients in the OAC1W treatment group, 218 in the OAC2W group and 224 in the omeprazole and amoxicillin group (fig 1).
Figure 1 Flow of participants through each phase of the study. ITT, intention to treat; OA, omeprazole; PP, per protocol.
Table 2 shows the eradication rates for the three groups according to ITT and PP analysis. There was no significant difference between OAC1W and OAC2W regimens in either ITT (p = 0.53) or PP (p = 0.71) analyses. As expected, both triple OAC treatments resulted in significantly higher eradication rates compared with omeprazole and amoxicillin treatment in both ITT and PP analyses (p<0.001). The difference in eradication rate between the OAC1W and OAC2W groups was 2% (95% CI –4% to 8%) in the ITT analysis, and 1.3% (95% CI –5.5% to 8.1%) in the PP analysis.
Table 2 H pylori eradication rates for each treatment regimen.
| ITT | PP | |||
|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | |
| OAC1W | 240/301 | 79.7 (74.8 to 83.9) | 183/219 | 83.6 (78.1 to 87.9) |
| OAC2W | 246/301 | 81.7 (77 to 85.7) | 185/218 | 84.9 (79.5 to 89.0) |
| OA | 136/305 | 44.6 (39.1 to 50.2) | 96/224 | 42.9 (36.5 to 49.4) |
ITT, intention‐to‐treat; PP, per protocol; OA, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and placebo for 2 weeks; OAC1W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 week, followed by omeprazole 20 mg twice daily and placebo for 1 week; OAC2W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 g twice daily for 2 weeks.
According to the ITT analysis, 235 of 301 patients in the OAC1W group (78.1%; 95% CI 73 to 82.3%), 243 of 301 patients in the OAC2W group (80.7%; 95% CI 75.9 to 84.8%), and 253 of 305 patients in the omeprazole and amoxicillin group (83.0%; 95% CI 78.3 to 86.7%) had healed ulcers after 2 weeks of treatment. According to the PP analysis, 200 of 227 patients in the OAC1W group (88.1%; 95% CI 83.2 to 91.7%), 212 of 233 patients in the OAC2W group (91.0%; 95% CI 86.6 to 94.1%) and 216 of 235 patients in the omeprazole and amoxicillin group (91.9%; 95% CI 87.7 to 94.7%) had healed ulcers after 2 weeks of treatment. As expected, there was no significant difference between the three regimens in terms of ulcer healing in either the ITT analysis (p = 0.31) or in the PP analysis (p = 0.35). A further 4 weeks of omeprazole treatment was given to patients without healed ulcers after 2 weeks and at the third visit, ulcer healing rates >90% were observed in all treatment groups in both the ITT and PP analyses.
The three treatments were well tolerated. The adverse events reported were mild or moderate and never severe. The proportions of patients experiencing adverse events was not significantly different in OAC1W and OAC2W groups, being 9.6% and 9.9% (p = 0.88), respectively (table 3). In the omeprazole and amoxicillin group, only 5.9% of patients experienced adverse events, but this was not significantly different from the other groups (p = 0.11). Similar proportions of patients from the three groups discontinued treatment due to adverse event (p = 0.48), as shown in table 3. The most frequent adverse events were diarrhoea, glossitis and stomatitis, as shown in table 4.
Table 3 Patients with adverse events and the frequency of withdrawal due to adverse events in the three treatment groups.
| OAC1W (n = 301), | OAC2W (n = 302), | OA (n = 305), | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Patients with AE | 29 (9.6) | 30 (9.9) | 18 (5.9) |
| Treatment discontinuation due to AE | 4 (1.3) | 5 (1.7) | 2 (0.7) |
AE, adverse event; OA, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and placebo for 2 weeks; OAC1W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 week, followed by omeprazole 20 mg twice daily and placebo for 1 week; OAC2W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 g twice daily for 2 weeks.
Table 4 Frequency of adverse events in the three treatment groups.
| Adverse event | OAC1W (n = 301), | OAC2W (n = 302), | OA (n = 305), |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Diarrhoea | 10 (3.3) | 10 (3.3) | 6 (2.0) |
| Glossitis | 7 (2.3) | 10 (3.3) | 3 (1.0) |
| Stomatitis | 4 (1.3) | 5 (1.7) | 2 (0.7) |
| Abdominal pain | 2 (0.7) | 4 (1.3) | 2 (0.7) |
| Allergic cutaneous reactions | 4 (1.3) | 2 (0.7) | 2 (0.7) |
| Dry mouth/throat | 3 (1) | 3 (1.0) | 0 |
| Tongue discoloration | 2 (0.7) | 1 (0.3) | 0 |
| Nausea | 0 | 2 (0.7) | 2 (0.7) |
| Vomiting | 0 | 1 (0.3) | 0 |
| Headache | 2 (0.7) | 2 (0.7) | 2 (0.7) |
| Monilia | 1 (0.3) | 1 (0.3) | 0 |
| Herpes simplex labialis | 0 | 1 (0.3) | 1 (0.3) |
OA, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and placebo for 2 weeks; OAC1W, omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 week, then omeprazole 20 mg twice daily and placebo for 1 week; OAC2W, omeprazole 20 mg twice daily + amoxicillin 1 g twice daily and clarithromycin 500 g twice daily for 2 weeks.
Discussion
There is still no general consensus regarding the optimal duration of triple therapy for H pylori eradication. European guidelines indicate that triple therapy for 1 week is acceptable7 whereas, in the USA, 10–14 days of treatment is still preferred.8,9 This difference in recommended treatment duration owes much to the fact that few studies have directly compared 1‐ and 2‐week triple treatment regimens. Of those that exist, almost all have been conducted in individual centres and with few patients.11,15,16,17,18,19,20,21 Most of these studies11,15,16,17,18,19,21 reported a trend towards better results with 2‐week eradication treatments, showing an increase in eradication rates of 4–11% in 2‐week when compared with 1‐week regimens. However, these increases were not statistically relevant. These trials also did not have the power to detect a difference of <15–20% due to the small numbers of patients enrolled. For example, Laine et al15 included 50 patients in each treatment group, but showed that 250 patients would be required to achieve statistical significance at the eradication rates (OAC1W = 86%; OAC2W = 92%; p = 0.11, by ITT analysis) achieved in their study. In a study in India, significantly higher rates of H pylori eradication were reported for a 2‐week triple treatment (95%) compared with a 1‐week regimen (54%).20 However, this study used a low dose of clarithromycin (250 mg twice daily), which could be responsible for the lower effectiveness of the 1‐week regimen. The general consensus is that, in a 1‐week PPI, clarithromycin and amoxicillin triple regimen, a twice daily dose of 500 mg clarithromycin is preferable.25
Just one multicentre study has compared the efficacy of 1 week and 2 weeks of triple therapy, but this study used metronidazole rather than clarithromycin in association with amoxicillin.26 Triple therapy with metronidazole and amoxicillin is recognised as having lower efficacy compared with PPI and clarithromycin‐based triple therapies and so it is not recommended by guidelines as part of triple therapy for H pylori eradication.7,8,9
The problem of small patient numbers has led some investigators to conduct meta‐analyses, which can be helpful because they represent a formal method for pooling large groups of patients from different studies. Two meta‐analyses have concluded that 2 weeks of triple therapy was significantly more effective than a 1‐week regimen, and reported an increase in the H pylori eradication rate of 9–12%.22,23 However, the results of both these meta‐analyses might have been biased by the fact that about half of the studies included had been published in abstract form.27,28,29,30 These abstracts do not report methods in detail and therefore an accurate evaluation of the quality of the study is not possible.31 One of the roles of meta‐analysis is to encourage better quality studies in the future and the authors of these two meta‐analyses suggested the need for studies that include many more patients.
Our study is the first large, multicentre, randomised, double blind, placebo‐controlled study directly comparing the efficacy of 1 and 2 weeks of triple therapy in patients with duodenal ulcer that is recommended by guidelines for H pylori eradication. We show that 1 week of treatment with omeprazole, clarithromycin and amoxicillin achieves H pylori eradication rates of 80% and 84% by ITT and PP analyses, respectively in duodenal ulcer patients, and that extending treatment to 2 weeks does not enhance eradication. The difference between eradication rates achieved with 1 and 2 weeks of triple therapy is not statistically significant.
The eradication rates reported in our study are consistent with data from other studies, which show that the efficacy of triple therapy for the eradication of H pylori has decreased in recent years.10,11,12 An increase in the prevalence of clarithromycin resistance seems to be the most important cause of this reduced efficacy.32 Novel agents and new and modified eradication regimens have been reported to improve eradication rates, but their efficacy still needs confirmation.33 One week of triple therapy remains the recommended first line treatment for H pylori eradication.
In our study, the tolerability of triple therapy was good and similar for both 1‐ and 2‐week regimens and was not significantly different from double therapy. It has been reported that increasing the duration of triple therapy decreases patient treatment compliance. However, the study reporting this finding could have been biased by the small number of patients included. We show that compliance was not significantly different for 1 and 2 weeks of triple therapy.
In conclusion, this study shows that the efficacy and safety of 1 week of triple therapy including amoxicillin and clarithromycin is not significantly different from 2 weeks of treatment for the eradication of H pylori in patients with duodenal ulcer.
Acknowledgements
We thank the following investigators: A Andriulli, Ospedale “Casa Sollievo della Sofferenza”, S Giovanni Rotondo (Foggia); G Bagnalasta, Ospedale Civile, Leno (Brescia); F Barberani, Ospedale S Camillo De Lellis, Rieti; A. Battocchia, Ospedale Borgo Trento, Verona; E Berrini, Ospedale “Endoli”, Angera (Varese); L Bonardi, Ospedale Gradenigo, Torino; M Bottari, Policlinico Universitario, Messina; L Buscarini Ospedale Civile, Piacenza; A Candela, Ospedale S Antonio Abate, Erice (Trapani); A Capria, Ospedale di Cisanello, Pisa; A Cardelli, Ospedale Civile degli Infermi, Rimini; F Catalano, Ospedale G Garibaldi, Catania; C Catanzaro, Policlinico Federico II, Napoli; L. Cipolletta, Ospedale “A. Maresca”, Torre del Greco (Napoli); E Colombo, Ospedale S. Corona, Garbagnate (Milano); F Confalonieri, Ospedale Civile, Sesto S Giovanni (Milano); C Cordiano, Ospedale Civile Maggiore, Verona; R Corinaldesi, Policlinico SOrsola, Bologna; F Costan, Ospedale Civile, Belluno; M Curzio, Ospedale di Circolo, Varese; S Dall'Acqua, Ospedale Civile, Gallarate (Milano); M D'Ayala Valva, Ospedale Civile “SS Annunziata”, Taranto; P Dal Monte, Ospedale Bellaria, Bologna; G De Filippo, Ospedale S. Leonardo, Salerno; A De Lio, Ospedale Civile, Praia a Mare (Cosenza); G Di Febo, Policlinico S Orsola, Bologna; S Di Matteo, Ospedale Spirito Santo, Pescara; P Di Maurizio, Ospedale Fatebenefratelli – Isola Tiberina, Roma; C Di Silverio, Ospedale Civile, Sanremo (Imperia); G Fatale, Azienda Ospedaliera S Maria, Terni; G Fava, Ospedale Civile, Bollate (Milano); A Ferrara, Ospedale Civile, Legnano (Milano); A Franzè, Ospedale Maggiore, Parma; M Frezza, Ospedale Civile, Gattinara (Treviso); L Gandolfi, Ospedale Malpighi, Bologna; M Giannelli, Ospedale S Camillo, Roma; A Giglio, Ospedale G Ciaccio, Catanzaro; V Giorgelli, Ospedale S Andrea, Vercelli; L Graziadei, Ospedale Civile S Carlo, Potenza; R Huscher, Ospedale di Vallemonica, Esine (Brescia); G Iaquinto, Ospedale S Giuseppe Moscati, Avellino; A Licata, Policlinico Universitario, Catania; R Lobello, Policlinico Universitario, Napoli; P Loriga, Ospedale “SS Trinità”, Cagliari; M Magalini, Ospedale Regionale “Ca Fondello”, Treviso; P Maiolo, Ospedale Civile, Forlì; G Malfitana, Ospedale degli Infermi, Biella; F Marotta, Ospedale S Anna, Camerlata (Como); L Melini Ospedale S Agostino, Modena; M Meloni, Ospedale “SS. Annunziata”, Sassari; G Menardo, Ospedale S Paolo, Savona; M Miglioli, Policlinico S Orsola, Bologna; G Minoli, Ospedale Valduce, Como; F Milesi, Ospedale Civile, Treviglio (Bergamo); F Molinari, Ospedale Civile, Alessandria; S Mosca, Ospedale Cardarelli, Napoli; F Pacini, Ospedale SLuca di Careggi, Firenze; P Paoluzzi, Policlinico Umberto I, Roma; A Pera, Ospedale Mauriziano, Torino; P Pignataro, Ospedale Fatebenefratelli, Milano; F Pino, Ospedale Villa Marina, Piombino (Grosseto); W Piubello, Presidio Ospedaliero di Desenzano, Salò (Brescia); A Prada, Ospedale Civile, Rho (Milano); C Prantera, Ospedale Nuovo Regina Margherita, Roma; G Rigo, Policlinico Universitario, Modena; G Rizzetto, Policlinico Le Molinette, Torino; A Russo, Policlinico Universitario, Catania; M Salvaneschi, Ospedale Civile, Stradella (Pavia); C Sategna‐Guidetti, Ospedale SGiovanni Battista, Torino; A Spadaccini, Ospedale Civile, Vasto (Chieti); M Spanedda, Ospedale Civile, Sassari; D Spotti, Ospedale S Carlo Borromeo, Milano; R Stabilini, Ospedale S Gerardo dei Tintori, Monza (Milano); A Trancanelli, Ospedale Silvestrini, Perugia; V Trimboli, Ospedale Civile, Cosenza; P Usai, Policlinico Universitario, Cagliari; M Valentini, Ospedale Civile, Aviano (Pordenone); A Waldthaler, Ospedale Civile, Bressanone (Bolzano). We also thank Dr Catherine Henderson and Dr Chris Winchester, who provided editorial assistance on behalf of AstraZeneca.
Abbreviations
13C‐UBT - carbon‐13 urea breath test
ITT - intention‐to‐treat
OAC1W - omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for 1 week, followed by omeprazole 20 mg twice daily and placebo for 1 week
OAC2W - omeprazole 20 mg twice daily and amoxicillin 1 g twice daily and clarithromycin 500 g twice daily for 2 weeks
PPI - proton pump inhibitor
Footnotes
Funding: This study was sponsored by AstraZeneca, Bracco SpA. and Malesci SpA.
Competing interests: RMZ has received reimbursement from Janssen‐Cilag and Abbot for attending symposia, and fees for speaking from AstraZeneca, Takeda and Abbott. GB‐P has received funds for research from Takeda (for a member of his staff) and from AstraZeneca (for publishing a book). RF has received fees for speaking from and has performed consultancy work for AstraZeneca. GG has received reimbursement for attending symposia. ER has received funds for organising education from Malesci and funds for consulting from AstraZeneca. FB has received fees for speaking from AstraZeneca and Takeda, reimbursement for organising education from Altana and has carried out consultancy work for AstraZeneca.
References
- 1.Bayerdorffer E, Miehlke S, Mannes G A.et al Double‐blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology 19951081412–1417. [DOI] [PubMed] [Google Scholar]
- 2.Ford A, Delaney B, Forman D.et al Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev 20044CD003840. [DOI] [PubMed] [Google Scholar]
- 3.Lind T, Veldhuyzen van Zanten S, Unge P.et al Eradication of Helicobacter pylori using one‐week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter 19961138–144. [DOI] [PubMed] [Google Scholar]
- 4.Veldhuyzen van Zanten S J, Bradette M, Farley A.et al The DU‐MACH study: eradication of Helicobacter pylori and ulcer healing in patients with acute duodenal ulcer using omeprazole based triple therapy. Aliment Pharmacol Ther 199913289–295. [DOI] [PubMed] [Google Scholar]
- 5.Lind T, Megraud F, Unge P.et al The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1‐week triple therapies. Gastroenterology 1999116248–253. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Bayerdorffer E, Diete U.et al The GU‐MACH study: the effect of 1‐week omeprazole triple therapy on Helicobacter pylori infection in patients with gastric ulcer. Aliment Pharmacol Ther 199913703–712. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O'Morain C.et al Current concepts in the management of Helicobacter pylori infection—the Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther 200216167–180. [DOI] [PubMed] [Google Scholar]
- 8.Peterson W L, Fendrick A M, Cave D R.et al Helicobacter pylori‐related disease: guidelines for testing and treatment. Arch Intern Med 20001601285–1291. [DOI] [PubMed] [Google Scholar]
- 9.Howden C W, Hunt R H. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998932330–2338. [DOI] [PubMed] [Google Scholar]
- 10.Katelaris P H, Forbes G M, Talley N J.et al A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology 20021231763–1769. [DOI] [PubMed] [Google Scholar]
- 11.Maconi G, Parente F, Russo A.et al Do some patients with Helicobacter pylori infection benefit from an extension to 2 weeks of a proton pump inhibitor‐based triple eradication therapy? Am J Gastroenterol 200196359–366. [DOI] [PubMed] [Google Scholar]
- 12.Vakil N, Lanza F, Schwartz H.et al Seven‐day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther 20042099–107. [DOI] [PubMed] [Google Scholar]
- 13.Calvet X, Ducons J, Bujanda L.et al Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. Am J Gastroenterol 20051001696–1701. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert J P, Dominguez‐Munoz A, Dominguez‐Martin A.et al Esomeprazole‐based therapy in Helicobacter pylori eradication: any effect by increasing the dose of esomeprazole or prolonging the treatment? Am J Gastroenterol 20051001935–1940. [DOI] [PubMed] [Google Scholar]
- 15.Laine L, Estrada R, Trujillo M.et al Randomized comparison of differing periods of twice‐a‐day triple therapy for the eradication of Helicobacter pylori. Aliment Pharmacol Ther 1996101029–1033. [DOI] [PubMed] [Google Scholar]
- 16.Louw J A, Van Rensburg C J, Moola S.et al Helicobacter pylori eradication and ulcer healing with daily lansoprazole, plus 1 or 2 weeks co‐therapy with amoxycillin and clarithromycin. Aliment Pharmacol Ther 199812881–885. [DOI] [PubMed] [Google Scholar]
- 17.Kiyota K, Habu Y, Sugano Y.et al Comparison of 1‐week and 2‐week triple therapy with omeprazole, amoxicillin, and clarithromycin in peptic ulcer patients with Helicobacter pylori infection: results of a randomized controlled trial. J Gastroenterol 199934(Suppl 11)76–79. [PubMed] [Google Scholar]
- 18.Moayyedi P, Langworthy H, Shanahan K.et al Comparison of one or two weeks of lansoprazole, amoxicillin, and clarithromycin in the treatment of Helicobacter pylori. Helicobacter 1996171–74. [DOI] [PubMed] [Google Scholar]
- 19.Dal Bò N, Di Mario F, Battaglia G.et al Low dose of clarithromycin in triple therapy for the eradication of Helicobacter pylori: one or two weeks? J Gastroenterol Hepatol 199813288–293. [DOI] [PubMed] [Google Scholar]
- 20.Bhasin D K, Sharma B C, Ray P.et al Comparison of seven and fourteen days of lansoprazole, clarithromycin, and amoxicillin therapy for eradication of Helicobacter pylori: a report from India. Helicobacter 2000584–87. [DOI] [PubMed] [Google Scholar]
- 21.de Silva H A, Hewavisenthi J, Pathmeswaran A.et al Comparison of one week and two weeks of triple therapy for the eradication of Helicobacter pylori in a Sri Lankan population: a randomised, controlled study. Ceylon Med J 200449118–122. [DOI] [PubMed] [Google Scholar]
- 22.Calvet X, Garcia N, Lopez T.et al A meta‐analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther 200014603–609. [DOI] [PubMed] [Google Scholar]
- 23.Ford A, Moayyedi P. How can the current strategies for Helicobacter pylori eradication therapy be improved? Can J Gastroenterol 200317(Suppl B)36B–40B. [DOI] [PubMed] [Google Scholar]
- 24.Bazzoli F, Zagari M, Pozzato P.et al Evaluation of short‐term low‐dose triple therapy for the eradication of Helicobacter pylori by factorial design in a randomized, double‐blind, controlled study. Aliment Pharmacol Ther 199812439–445. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert J P, Gonzalez L, Calvet X.et al Proton pump inhibitor, clarithromycin and either amoxycillin or nitroimidazole: a meta‐analysis of eradication of Helicobacter pylori. Aliment Pharmacol Ther 2000141319–1328. [DOI] [PubMed] [Google Scholar]
- 26.Wong B C, Xiao S D, Hu F L.et al Comparison of lansoprazole‐based triple and dual therapy for treatment of Helicobacter pylori‐related duodenal ulcer: an Asian multicentre double‐blind randomized placebo controlled study. Aliment Pharmacol Ther 200014217–224. [DOI] [PubMed] [Google Scholar]
- 27.Katicic M, Presecki V, Marusic M.et al Eradication of H. pylori infection in peptic ulcers with four different drug regimens. Gut 199639A144 [Google Scholar]
- 28.Dammann H G, Folsch U R, Hahn E G.et al 7 vs 14 day treatment with pantoprazole, clarithromycin and metronidazole for cure of H. pylori infection in duodenal ulcer patients. Gut 199741A95. [DOI] [PubMed] [Google Scholar]
- 29.Paoluzi P, Rossi P, Consolazio A. A single‐blind monocentric study of four treatments for H. pylori eradication: an interim report, Gastroenterology 1998114G1039 [Google Scholar]
- 30.Paoluzi P, Iacopini F, Consolazio A. Two week PPI‐based triple therapy with amoxicillin and clarithromycin has a higher efficacy in Helicobacter pylori eradication. Gut 200149A2545 [Google Scholar]
- 31.Cho M K, Bero L A. Instruments for assessing the quality of drug studies published in the medical literature. JAMA 1994272101–104. [PubMed] [Google Scholar]
- 32.Mègraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004531374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bytzer P, O'Morain C. Treatment of Helicobacter pylori. Helicobacter 200510(Suppl 1)40–46. [DOI] [PubMed] [Google Scholar]