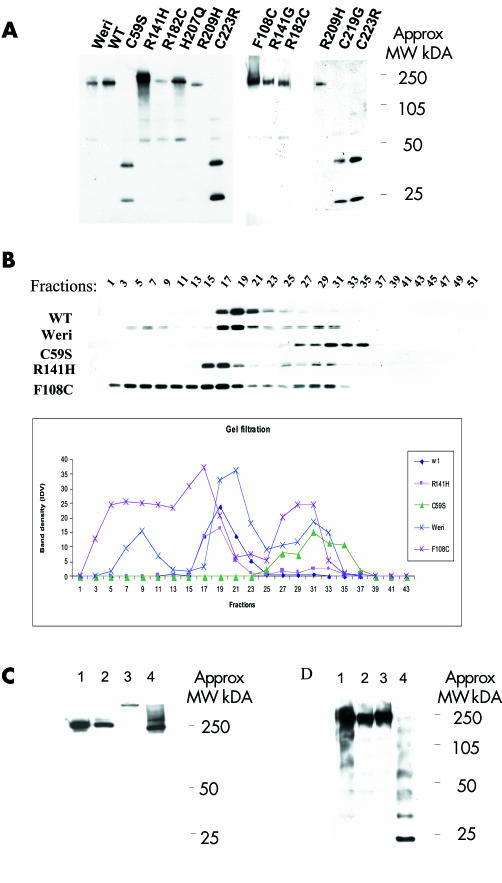
Figure 4 Non‐reducing gel and gel filtration for secreted wild type (WT) retinoschisin, retinoschisin mutants, and Weri‐Rb1 Retinoschisin. (A) Non‐reducing gel for WT and mutant retinoschisin showing oligomerisation of WT and mutants. Three of the mutants (C59S, C219G, and C223R) formed only monomers and dimers. (B) Gel filtration analysis using concentrated cell culture media from Weri‐Rb1 cells, stably transfected wild type RS1 and C59S, F108C and R141H mutants. 50 fractions were collected. The results of western blot analysis are shown from alternate fractions in the upper panel and indicate WT retinoschisin (and R141H) forms oligomers. C59S is detected in later fractions consistent with monomers, whereas F108C is eluted in earlier fractions indicating a greater tendency to oligomerisation than WT. The lower panel depicts the same data in graph form. (C) Non‐reducing gel analysing fractions from gel filtration. Lanes 1 and 2 show fractions 19 from Weri‐Rb1 cells and COS‐7 cells stably expressing WT retinoschisin respectively and lanes 3 and 4 show fractions 5 and 17 from COS‐7 cells stably expressing F108C retinoschisin, respectively. F108C mutant retinoschisins from fraction 5 runs slower than the other fractions indicating a larger sized multimer. (D) Non‐reducing gel demonstrating the effects of heating and NEM treatment on the WT retinoschisin. Each lane contains conditioned WT cell medium. Lane 1 unheated and untreated, lane 2 treated with NEM in 4M urea at room temperature, lane 3 treated with NEM in 4M urea and heated to 100°C for 3 minutes, lane 4 heated to 100°C for 3 minutes without NEM or urea. Laddering indicating dissociation of the oligomer is seen in lane 4 only.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.