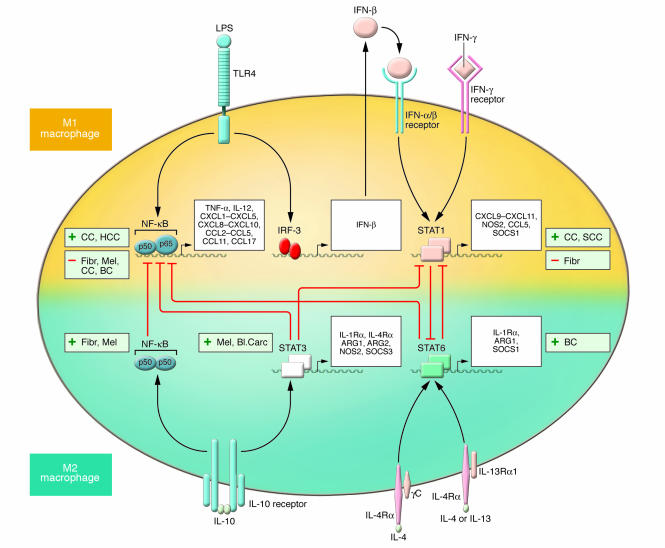
Figure 3. Molecular pathways of macrophage polarization and their role in tumor progression.
The major pathways of macrophage polarization and current evidence linking their activation with either tumor progression (+) or regression (–) are outlined. The overall view suggests that M2 macrophage–polarizing signals (such as IL-10, IL-4, and IL-13) are mainly associated with tumor progression. Contrasting evidence associates M1 macrophage–polarizing pathways (such as IFN-γ and TLR ligation) with either tumor progression or regression. The crosstalk between the M1 and M2 macrophage–polarizing pathways, which results in reciprocal modulation, are also indicated. As shown, IL-10–mediated induction of the p50 NF-κB homodimer interferes with NF-κB activation and M1 macrophage–induced inflammation. The balance between activation of M1 macrophage–associated STAT1 and M2 macrophage–associated STAT3 and STAT6 finely regulates macrophage polarization and activity. A predominance of NF-κB and STAT1 activation results in M1 macrophage polarization, which promotes cytotoxic and inflammatory functions. In contrast, a predominance of STAT3 and STAT6 activation results in M2 macrophage polarization, which is associated with immune suppression and tumor progression. As discussed in the text, IL-23 might also contribute to the polarization decision as it activates different STATs, including STAT1 and STAT3, in TAMs, but direct evidence is missing. CC, colorectal carcinoma; HCC, hepatocarcinoma; Fibr, fibrosarcoma; Mel, melanoma; BC, breast carcinoma; SCC, squamous cell carcinoma; Bl. Carc, bladder carcinoma.