Fig. 2. Peptide cleavage of DNA catalyzed by Flp variants.
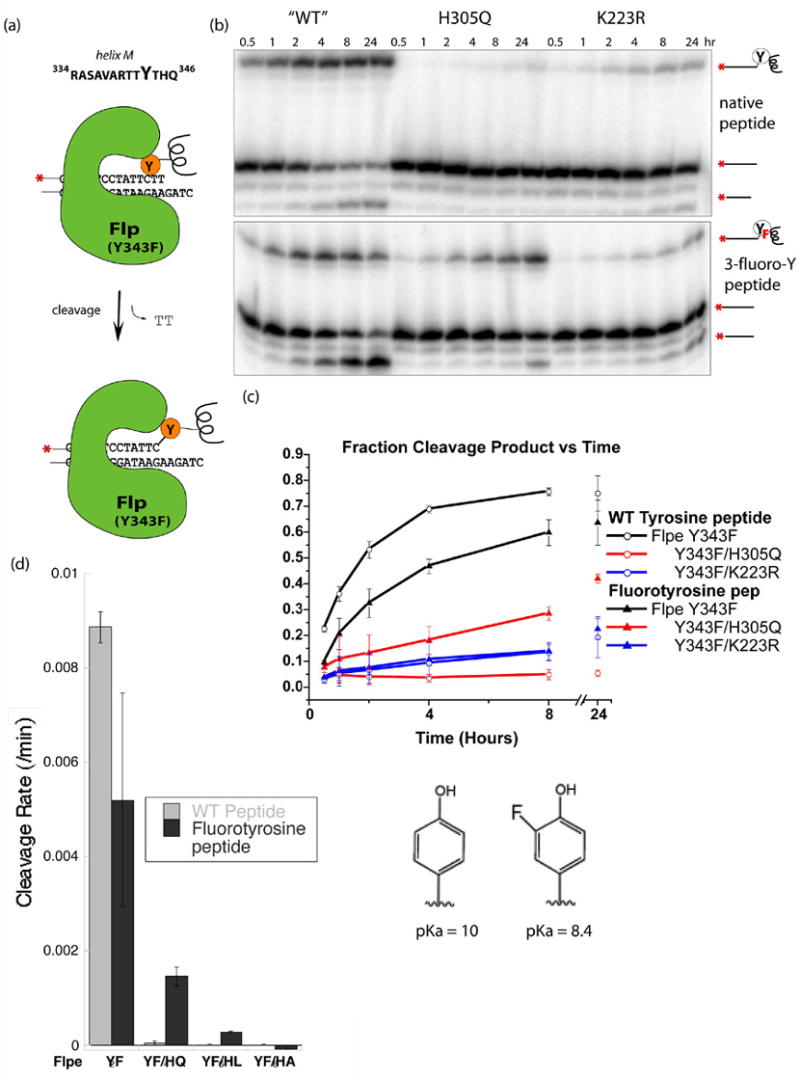
(a) Schematic describing the cleavage reaction with a 13 amino acide peptide corresponding to residues 334–346 of Flp, including helix M and the flanking loops. Peptides bearing tyrosine or 3-fluorotyrosine were reacted with Flp variants and a DNA suicide substrate containing a single Flp binding site which releases a TT dinucleotide upon cleavage. (b) The products of the cleavage reactions separated on sequencing gels. Hydrolysis of the phosphotyrosine linkage happens over time, especially with the fluorinated tyrosine analog. (c) Plot of Product vs. time. Product, shown as a percentage of total DNA, is the sum of the DNA-peptide complex and the subsequent hydrolysis product. The activity of both the Y343F and K223R decreased when the fluorotyrosine-containing peptide was used. The activity of H305Q, however, showed a significant increase. (d) Cleavage rates with several different Flpe H305 mutants.
