Fig. 2.
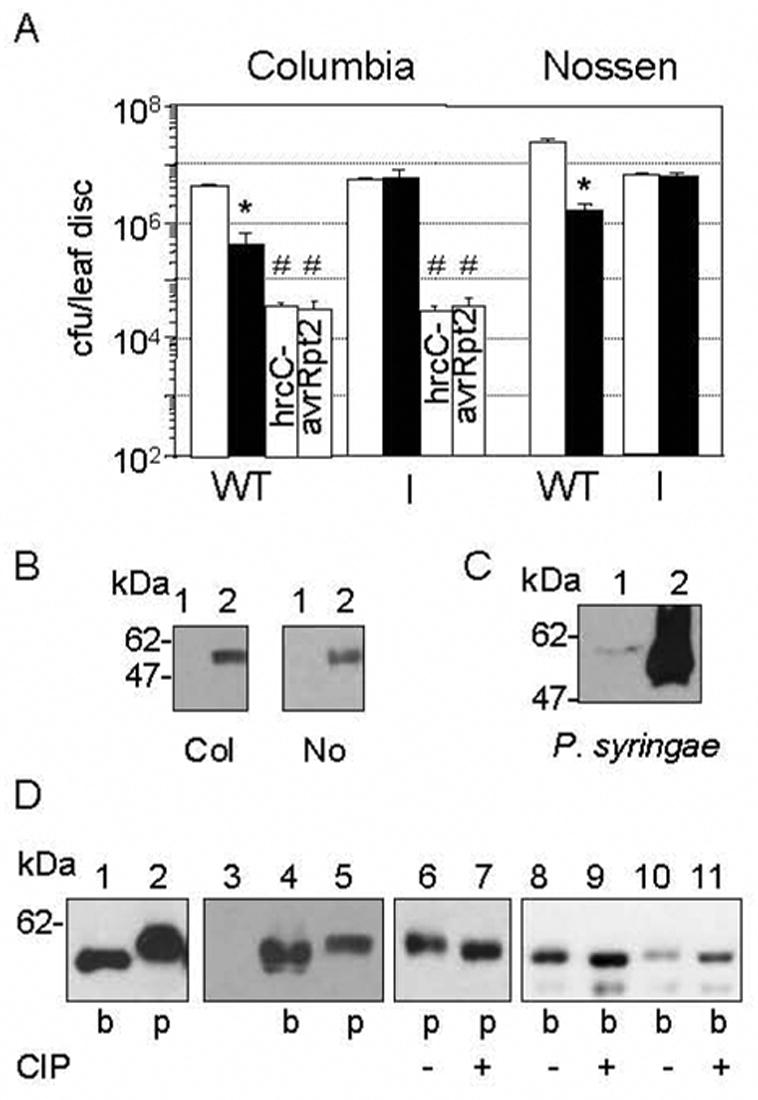
HopI1 functions inside plant cells and is phosphorylated.
(A) Col and No plants constitutively expressing HopI1PmaES4326 (I, JJ30) rescued the virulence defect of the ΔhopI1 PmaES4326 strain (black bar). Col with or without HopI1 had the same susceptibility to hrcC− PmaES4326 or PmaES4326/avrRpt2 (#P>0.4). (*The growth of the ΔhopI1 strain was lower than PmaES4326 strain (white bar) on wild-type plants, P<0.0001). Similar results were obtained with 2 additional, independently derived transgenic HopI1-expressing Col and No lines (not shown).
(B) HopI1 was expressed in transgenic A. thaliana Col (left panel) and No (right panel). Proteins were detected by SDS-PAGE and Western blot analysis with anti-HA antibody. Lines: (1) Wild-type plants, (2) Transgenic plants constitutively expressing HopI1 (JJ30=hopI1PmaES4326-HA-Myc-His).
(C) HopI1-HA-Myc-His expressed from the native promoter and detected with anti-HA antibody exhibited an apparent increase in molecular mass when PmaES4326/JJ19 was grown in A. thaliana (1) compared to hrp-inducing conditions in vitro (2) for 16 h.
(D) HopI1 phosphorylation in bacteria and plants. HopI1PmaES4326-HA-Myc-His expressed in bacteria (b, PmaES4326/JJ19) and transgenic plants (p, Col/JJ30) was detected with anti-HA antibody. Lanes: (1–5) HopI1 expressed in plants had an apparent greater mass than in bacteria. (3) Control A. thaliana Col; (4) The different masses of HopI1 in plants did not result from abundant plant protein displacing the HopI1 species, since when PmaES4326/JJ19 extract was mixed with Col extract (4), HopI1 mass appeared lower than when expressed in plants (5, Col/JJ30). (6–11) HopI1 was phosphorylated in plants and probably in bacteria. Extracts from plants (p) and bacteria (b) were incubated without (−) or with (+) CIP phosphatase. Dephosphorylation of HopI1 results in faster migration in the polyacrylamide gel. (10,11) PmaES4326/JJ19 extract mixed with Col extract. The shift in the HopI1 species in the plant extract was not caused by dephoshorylation of a comigrating abundant plant protein because bacterial-expressed protein incubated with CIP had the same apparent mass both in bacterial protein extract alone (9) or bacterial protein extract mixed with wild-type Col extract (11).
