Fig. 2.
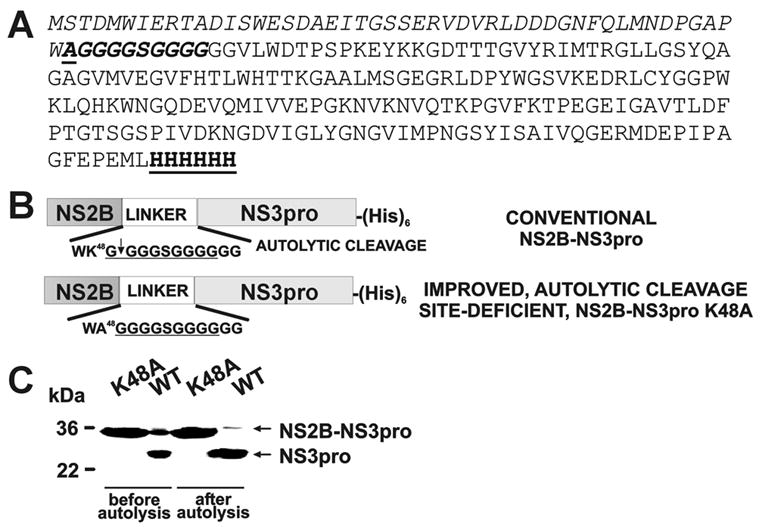
NS2B-NS3pro and NS2B-NS3pro K48A constructs. (A) The peptide sequence of the WNV NS2B-NS3pro K48A construct. The N-terminal NS2B sequence is italisized. The linker sequence with the K48A mutation (underlined) is italisized and bolded. The NS3pro is shown in a regular font. The C-terminal Hisx6 tag is bolded and underlined. (B) The 48 amino acid residue-long, central portion of NS2B (NS2B) was linked with the NS3pro sequence via a GGGGSGGGG linker (in the figure, the linker sequence is underlined). The constructs were C-terminally tagged with a (His)x6 tag. After the isolation, the NS2B-NS3pro construct autolytically cleaved the G49↓G50 bond and generated the individual NS3pro domain commencing at Gly50. To inactivate the autolytic cleavage site Ala was substituted for Lys48 in the C-terminal region of the NS2B moiety. Because of the furin-like cleavage specificity of the WNV NS2B-NS3pro, the presence of either Lys or Arg at the P2 position is essential for substrate cleavage by the protease. (C) Autolytic conversion of the NS2B-NS3pro construct. The purified NS2B-NS3pro (WT; wild type) and NS2B-NS3pro K48A (K48A) samples were each incubated overnight (0.1 mg/ml; pH 8, 24°C) to generate the NS3pro domain. The digest samples were analyzed by SDS gel electrophoresis followed by Coomassie staining of the gel. In contrast to WT, K48A is resistant to self-proteolysis.
