Abstract
Aim
To evaluate the efficacy of pars plana vitrectomy (PPV) in the management of chronic uveitic cystoid macular oedema (CMO).
Methods
A prospective, interventional, randomised, controlled, pilot study. 23 eyes of 23 patients with CMO secondary to chronic intermediate or posterior uveitis unresponsive to medical treatment were randomised into a surgical (group S) or medical group (group M). 12 patients in group S underwent PPV as opposed to 11 patients in group M who received systemic corticosteroid and/or immunosuppressive treatment during the study period. The primary outcome measures of the study were change in visual acuity and angiographic appearance of CMO at 6 months.
Results
Mean visual acuity in group S improved significantly from 1.0 (0.62) at baseline to 0.55 (0.29) at 6 months following vitrectomy (p = 0.011), with five (42%) eyes reaching vision of 20/40 or better. Conversely, mean visual acuity in group M improved only marginally by 0.03 (0.27) (p = 0.785). CMO after vitrectomy was angiographically improved in four (33%) eyes, remained unchanged in seven (58%) eyes, and deteriorated in one (8%) eye. In the medical group, fluorescein leakage decreased in one eye, did not alter in four eyes, and deteriorated in two eyes.
Conclusion
PPV for macular oedema secondary to chronic uveitis despite angiographic improvement in only one third of the patients, seems to have a significant beneficial effect on visual function. This study provides enough evidence to justify a large scale trial which would define the role of vitrectomy in uveitic macular oedema.
Keywords: pars plana vitrectomy, cystoid macular oedema, uveitis, pilot study
Recent advances in the vitreoretinal surgery have expanded the indications of vitrectomy in a broad spectrum of diseases.1 Cystoid macular oedema (CMO) is a common complication of chronic uveitis resulting in significant reduction of visual acuity in 21–52% of patients.2 Despite rigorous treatment with steroids and other immunomodulators, long term results indicate that macular oedema may persist in a substantial number of cases.3 The role of vitreous surgery in uveitic macular oedema is uncertain, although, there have been sporadic reports on the outcome of pars plana vitrectomy (PPV) in cases unresponsive to medical treatment.1,3,4,5,6,7,8,9,10
The purpose of this pilot study was to prospectively evaluate the therapeutic effect of PPV on patients with chronic intermediate or posterior uveitis and secondary macular oedema refractory to systemic immunosuppression.
Patients and methods
Twenty three patients with CMO secondary to chronic intermediate or posterior uveitis were recruited prospectively between October 1998 and June 2003. Eligibility criteria were CMO unresponsive to medical treatment (systemic steroids and/or other immunosuppressive agents) after 3 months, uveitis controlled medically with respect to inflammatory activity, no other coexisting macular pathology, and no previous vitreoretinal surgery.
Baseline examination included measurement of best corrected Snellen visual acuity and dilated slit lamp biomicroscopy. The inflammatory activity of the anterior chamber and vitreous was graded on a 0–4 scale.11,12 Goldmann applanation tonometry was also performed and dilated funduscopy confirmed the status of CMO.
All recruits had fundus fluorescein angiogram (FFA), which was evaluated by a masked reader and graded using the classification system described by Yannuzzi.13 Subsequently, participants were randomly assigned into a surgical (group S) or medical group (group M). In order to ensure a closer balance in the treatment allocation, permuted block randomisation was the preferred method of participants' assignment14 in the two groups. Randomisation status was provided by the study statistician and examiners were unaware of it.
Patients in the surgical group underwent standard three port PPV under local or general anaesthesia. All surgical patients had a short course of oral prednisolone before vitrectomy, which was weaned off to preoperative levels 3–6 weeks after surgery. Baseline examination was repeated 1 week and 1, 3, and 6 months following surgical intervention. Fundus fluorescein angiography (FFA) was performed 1 month and 6 months postoperatively. Patients in the control group (medical) underwent the same assessment at the same time points as the surgical participants.
The primary outcome measures of the study were change in visual acuity and angiographic appearance of CMO at 6 months. At the final follow up the minimum difference from baseline to be considered as significant was two Snellen lines of change in visual acuity and a change in grade on masked reading of the FFA.
Snellen visual acuity was converted to logMAR for the statistical analysis. Relations between categorical variables were evaluated using χ2 test. Changes in visual acuity, FFA score, intraocular pressure and number of systemic anti‐inflammatory agents at the end of the follow up period were analysed with the Wilcoxon signed rank test. All tests of association were considered to be statistically significant if p<0.05. Analyses were carried out using SPSS (version 10.0, SPSS Inc, Chicago, IL, USA).
Results
Following randomisation 12 patients underwent PPV (group S) and 11 subjects were randomised to the medical group and treated conservatively with systemic anti‐inflammatory and/or immunosuppressive agents (group M). Clinical characteristics of all patients are summarised in table 1.
Table 1 Baseline characteristics of recruits (21 eyes of 21 patients) in the medical (n = 11) and surgical (n = 12) group.
| Medical | Surgical | |
|---|---|---|
| Age (years) | ||
| Range | 29–61 | 20–70 |
| Mean (SD) | 45 (12) | 47 (12) |
| Sex | ||
| Male | 6 (55%) | 7 (58%) |
| Female | 5 (45%) | 5 (42%) |
| Aetiology of uveitis | ||
| Idiopathic | 9 (82%) | 8 (67%) |
| Sarcoidosis | 1 (9%) | 1 (8%) |
| Multiple sclerosis | 1 (9%) | 1 (8%) |
| Leishmaniasis | 1 (8%) | |
| Seronegative arthritis | 1 (8%) | |
| Lens status | ||
| Phakic | 1 (9%) | 1 (8%) |
| Pseudophakic | 10 (91%) | 11 (92%) |
| LogMAR visual acuity | ||
| Range | 0.3–2.3 | 0.5–2.0 |
| Mean (SD) | 0.95 (0.65) | 0.99 (0.59) |
Functional outcome
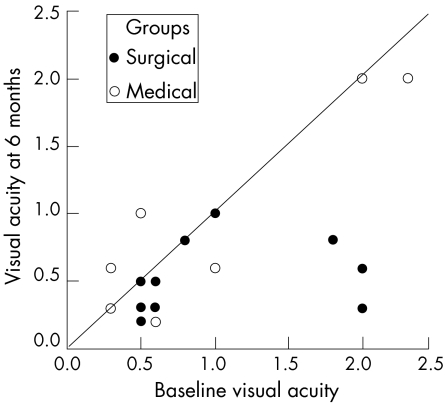
Baseline visual acuity in group S and group M was 1.0 (0.62) and 0.95 (0.65), respectively (p = 0.843). Six months following PPV, mean visual acuity in group S improved significantly to 0.55 (0.29) (p = 0.011) with five (42%) eyes reaching vision of 20/40 or better (fig 1, table 2). Six (50%) patients experienced significant improvement in visual acuity of two or more Snellen lines. Conversely, mean visual acuity in control eyes (group M) improved marginally by 0.03 (0.27) (p = 0.785). Improvement of two or more lines was observed in two (18%) patients, there was no change in six (54%) patients, and in two (18%) eyes vision decreased by one or more lines. Despite better visual outcome in patients undergoing vitrectomy, subgroup analysis showed no statistically significant difference (p = 0.131) between the two groups regarding visual improvement of two or more lines.
Figure 1 Scatter plot showing visual acuity at baseline and at the end of the study period for patients who underwent vitrectomy and control eyes.
Table 2 Demographic and clinical data of all study participants.
| No | Age (years) | Eye | Underlying diseases | Group | Visual acuity | Change in FFA score* | Systemic treatment | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | ||||||
| 1 | 61 | LE | None | M | 20/60 | 20/60 | 0 | None | None |
| 2 | 45 | RE | Sarcoidosis | M | 20/60 | 20/200 | steroids | steroids† | |
| 3 | 50 | RE | MS | M | CF | CF | steroids | None | |
| 4 | 35 | RE | None | M | 20/200 | 20/200 | 0 | None | None |
| 5 | 31 | LE | None | M | 20/40 | 20/80 | steroids | None | |
| 6 | 51 | RE | None | M | 20/40 | 20/40 | 0 | None | None |
| 7 | 57 | RE | None | M | 20/200 | 20/200 | 0 | None | None |
| 8 | 47 | LE | None | M | 20/200 | 20/200 | 1 | steroids | steroids† |
| 9 | 29 | RE | None | M | HM | CF | steroids | steroids† | |
| 10 | 55 | RE | None | M | 20/80 | 20/30 | −1 | None | None |
| 11 | 30 | LE | None | M | 20/200 | 20/80 | 1 | None | Aciclovir |
| 12 | 49 | RE | None | S | 20/80 | 20/60 | −1 | steroids/CsA | steroids↓ |
| 13 | 70 | RE | None | S | 10/600 | 20/120 | 0 | None | None |
| 14 | 54 | LE | None | S | CF | 20/80 | 0 | CsA | None |
| 15 | 44 | LE | None | S | 20/60 | 20/40 | 0 | steroids/CsA | steroids/CsA |
| 16 | 49 | RE | Leishmaniasis | S | 20/60 | 20/30 | −1 | steroids/Aza | steroids/Aza |
| 17 | 52 | RE | Sarcoidosis | S | 20/80 | 20/40 | 0 | None | None |
| 18 | 20 | LE | None | S | CF | 20/40 | 0 | None | None |
| 19 | 50 | RE | MS | S | 20/200 | 20/200 | 0 | None | None |
| 20 | 33 | RE | SA | S | 20/200 | 20/200 | −2 | None | None |
| 21 | 41 | LE | None | S | 20/60 | 20/60 | 0 | None | None |
| 22 | 54 | RE | None | S | 20/80 | 20/40 | −1 | None | None |
| 23 | 50 | RE | None | S | 20/120 | 20/120 | 1 | Aza | Aza |
FFA, fundus fluorescein angiogram; MS, multiple sclerosis; SA, seronegative arthritis; CF, counting fingers; HM, hand motion; CsA, ciclosporin A; Aza, azathioprine
*FFA score = score at 6 months − baseline score.
†Reduction in dose.
Angiographic outcome
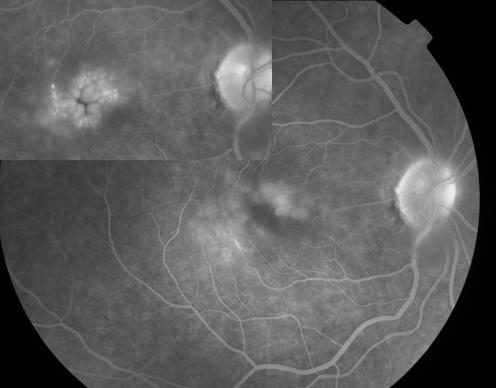
Six months following pars plana vitrectomy, CMO was angiographically improved in four (33%) eyes (fig 2) with complete absence of fluorescein leakage in two of them. Macular oedema remained unchanged in seven (58%) eyes and showed evidence of increased leakage on the fluorescein angiogram in one (8%) eye.
Figure 2 Composite of fluorescein angiograms showing reduction of fluorescein leakage in macular oedema following pars plana vitrectomy, compared to preoperative status (top left).
In the medical group, good quality fluorescein angiograms were obtained in only seven of 11 cases. Fluorescein leakage decreased in one eye, did not alter in four eyes, and deteriorated in two eyes.
Analysis of the angiographic appearance of macular oedema at the end of the follow up, showed no statistical difference (p>0.05) compared to baseline scores for either surgical or medical groups (Wilcoxon signed rank test).
Systemic medical treatment and course of uveitis (table 2)
All patients had been treated with differing courses of anti‐inflammatory agents before the recruitment. At the time of randomisation five patients in each group were receiving systemic medical treatment. Six months following vitrectomy the systemic medications were reduced in two patients, whereas in three patients the systemic anti‐inflammatory and/or immunosuppressive regimen did not alter. In the medical group systemic steroids were reduced in three eyes, remained unchanged in two eyes, while one eye required the introduction of systemic antiviral treatment.
There was no significant difference (p = 0.948) between the two groups with respect to the change in the anterior chamber activity. The latter improved marginally by 0.10 (0.32) in the medical group and by 0.21 (0.20) in patients who underwent PPV. Conversely, vitritis was significantly less (p = 0.016) following surgical intervention. At the 6 month follow up, vitreous activity had improved by 1.45 (1.12) in the surgical group as opposed to 0.27 (0.64) in control eyes.
Complications of surgery
There were no significant intraoperative or postoperative complications following vitrectomy in the surgical group. One peripheral retinal break was treated with application of cryotherapy. Two patients had transient ocular hypertension, which was treated with topical antiglaucoma agents for 1 month.
Discussion
Cystoid macular oedema is the most common cause of blindness and visual impairment in uveitic patients. It is usually the sequel of chronic intraocular inflammation and its incidence varies according to the underlying clinical syndrome.
Although the therapeutic approach of uveitic CMO is controversial, periocular intravitreal or systemic steroids and/or immunosuppressive agents are often the treatment of choice.15,16,17,18,19
PPV has recently been utilised as a potentially effective treatment modality in inflammatory CMO unresponsive to medical therapy4,7,8
In our cohort of patients with CMO secondary to chronic uveitis, vision improved by two or more lines in 50% of the eyes undergoing vitrectomy as opposed to only 18% of the eyes in the control group. In addition angiographic improvement of CMO was observed in 33% of the vitrectomised eyes compared to 14% in the medical group. The small sample size of this pilot study did not allow these results to reach statistical significance. Nevertheless, our study demonstrates the potential of vitrectomy to improve visual acuity in uveitic CMO.
The possible mechanisms of regression of macular oedema after PPV remain uncertain. There is some evidence that removal of inflammatory mediators from the vitreous gel may have a therapeutic effect on the CMO as it may result in reduced antigen presentation and increased responsiveness to systemic treatment.20,21,22
Mechanical factors may also have a role in the pathogenesis of uveitic CMO. Previous reports of eyes with peripheral uveitis and posterior vitreous adhesion document a higher incidence and more refractory macular oedema compared to eyes with complete vitreoretinal separation.23
In our pilot series the effect of PPV on the angiographic outcome of CMO appeared to be modest with 66% of the eyes showing no improvement after 6 months of follow up. Since removal of the vitreous may have improved vision because of improvement in media clarity rather than specifically inducing changes to pre‐existing CMO a larger scale study will be necessary to determine the relative contributions of these potential mechanisms.
The limitations of this pilot study include the small sample size, the use of Snellen chart for assessment of visual acuity, and the relatively short duration of follow up. Patients who underwent pars PPV had a short course of oral steroids, which could potentially introduce bias, as the outcome may not be clearly attributed to the surgery. However since all patients had chronic CMO, refractory to previous long standing systemic treatment, it is probably unlikely that the short course of steroids could have a substantial effect on the functional and anatomic results of the study.
In conclusion, this is the first randomised controlled study to investigate prospectively the controversial role of vitrectomy on uveitic CMO. The results we present suggest that PPV may have a beneficial effect on visual function of patients with macular oedema secondary to chronic uveitis. Visual recovery is not necessarily accompanied by angiographic improvement of CMO; however, it was found to be significant compared to preoperative levels. Despite the absence of robust data as with other pilot studies, this survey provides enough evidence to justify a large scale trial which would have the potential to define the role of vitrectomy on uveitic macular oedema.
Abbreviations
CMO - cystoid macular oedema
FFA - fundus fluorescein angiogram
PPV - pars plana vitrectomy
Footnotes
Financial interest: none.
The study was approved by the local research ethics committee at Moorfields Eye Hospital, London.
References
- 1.Aylward G W. The place of vitreoretinal surgery in the treatment of macular oedema. Doc Ophthalmol 199997433–438. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman P L. Pars planitis and other intermediate uveitis. In: Yanoff M, Duker JS, eds. Ophthalmology. St Louis: Mosby, 199910.1–10.6.
- 3.Heiligenhaus A, Bornfeld N, Wessing A. Long‐term results of pars plana vitrectomy in the management of intermediate uveitis. Curr Opin Ophthalmol 1996777–79. [DOI] [PubMed] [Google Scholar]
- 4.Heiligenhaus A, Bornfeld N, Foerster M H.et al Long‐term results of pars plana vitrectomy in the management of complicated uveitis. Br J Ophthalmol 199478549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiryu J, Kita M, Tanabe T.et al Pars plana vitrectomy for cystoid macular oedema secondary to sarcoid uveitis. Ophthalmology 20011081140–1144. [DOI] [PubMed] [Google Scholar]
- 6.Freeman G, Matos K, Pavesio C E. Cystoid macular oedema in uveitis: an unsolved problem. Eye 20011512–17. [DOI] [PubMed] [Google Scholar]
- 7.Androudi S, Ahmed M, Fiore T.et al Combined pars plana vitrectomy and phacoemulsification to restore visual acuity in patients with chronic uveitis. J Cataract Refract Surg 200531472–478. [DOI] [PubMed] [Google Scholar]
- 8.Radetzky S, Walter P, Fauser S.et al Visual outcome of patients with macular oedema after pars plana vitrectomy and indocyanine green‐assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol 2004242273–278. [DOI] [PubMed] [Google Scholar]
- 9.Dugel P U, Rao N A, Ozler S.et al Pars plana vitrectomy for intraocular inflammation‐related cystoid macular oedema unresponsive to corticosteroids. A preliminary study. Ophthalmology 1992991535–1541. [DOI] [PubMed] [Google Scholar]
- 10.Wiechens B, Nolle B, Reichelt J A. Pars‐plana vitrectomy in cystoid macular oedema associated with intermediate uveitis. Graefes Arch Clin Exp Ophthalmol 2001239474–481. [DOI] [PubMed] [Google Scholar]
- 11.Hogan M J, Kimura S J, Thygeson P. Signs and symptoms of uveitis. I. Anterior uveitis. Am J Ophthalmol 195947155–170. [DOI] [PubMed] [Google Scholar]
- 12.Nussenblat R B, Palestine A G, Chan C C.et al Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 198592467–471. [DOI] [PubMed] [Google Scholar]
- 13.Yannuzzi L A. A perspective on the treatment of aphakic cystoid macular oedema. Surv Ophthalmol 198428(Suppl)540–553. [DOI] [PubMed] [Google Scholar]
- 14.Matts J P, Lachin J M. Properties of permuted‐block randomization in clinical trials. Controlled Clin Trials 19889327–344. [DOI] [PubMed] [Google Scholar]
- 15.Tessler H, Lam S. Cystoid macular oedema. In: Pepose JS, Holland GN, Wilhelmus KR, eds. Ocular infection and immunity. St Louis: CV Mosby, 1996553–589.
- 16.Jennings T, Russin M M, Tessler H H.et al Posterior subtenon's injections of triamcinolone acetonide in uveitis patients with cystoid macular oedema. Jpn J Ophthalmol 199832385–391. [PubMed] [Google Scholar]
- 17.Yoshikowa K, Kotake S, Ichiishi A.et al Posterior subtenon's injections of repository corticosteroids in uveitis patients with cystoid macular oedema. Jpn J Ophthalmol 19953971–76. [PubMed] [Google Scholar]
- 18.Tranos P G, Wickremasinghe S S, Stangos N T.et al Macular oedema. Surv Ophthalmol 200449470–490. [DOI] [PubMed] [Google Scholar]
- 19.Dick A D. The treatment of chronic uveitic macular oedema. Br J Ophthalmol 1994781–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liversidge J, Dick A, Cheng Y F.et al Retinal antigen specific lymphocytes, TCR‐gamma delta T cells and CD5+ B cells cultured from the vitreous in acute sympathetic ophthalmitis. Autoimmunity 199315257–266. [DOI] [PubMed] [Google Scholar]
- 21.Moller D R. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 19991624–31. [PubMed] [Google Scholar]
- 22.Muhaya M, Calder V L, Towler H M.et al Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol 1999116410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hikichi T, Trempe C L. Role of the vitreous in the prognosis of peripheral uveitis. Am J Ophthalmol 1993116401–405. [DOI] [PubMed] [Google Scholar]