Abstract
Aim
To determine whether intravitreal injection of tacrolimus suppresses ongoing experimental autoimmune uveoretinitis (EAU) in rats.
Methods
Rats were immunised with interphotoreceptor retinoid‐binding protein peptide (R14) and given an intravitreal injection of tacrolimus on day 12 after immunisation. Intraocular inflammation was assessed by slit‐lamp biomicroscopy and histopathological examination. Interferon γ and tumour necrosis factor α protein levels in the ocular tissues were measured. Gene expression of chemokines was determined in ocular tissues by reverse transcription‐polymerase chain reaction. To evaluate the systemic effect of intravitreal injection of tacrolimus, delayed‐type hypersensitivity was measured by ear swelling.
Results
Clinical and pathological scores showed that ocular inflammation of tacrolimus‐treated eyes was markedly less than that of vehicle‐treated eyes. The amount of interferon γ and tumour necrosis factor α was considerably inhibited in tacrolimus‐treated eyes. The gene expression of monocyte chemattractant protein‐1 (MCP‐1) and regulated upon activation, normal T cell expressed and secreted (RANTES) was markedly reduced in tacrolimus‐treated eyes. Delayed‐type hypersensitivity responses were not impaired in tacrolimus‐treated rats.
Conclusions
Intravitreal injection of tacrolimus was highly effective in suppressing the ongoing process of EAU without any side effects on systemic cellular immunity. This treatment may be useful in the management of patients with severe uveitis.
Experimental autoimmune uveoretinitis (EAU) is an inflammatory disease model that shares many clinical and histological features with human uveitis such as Behçet's disease, Vogt–Koyanagi–Harada disease and sarcoidosis.1,2,3 In EAU‐susceptible strains of rat, immunisation with retinal antigen such as S‐antigen or bovine interphotoreceptor retinoid‐binding protein peptide induces T helper cell 1‐mediated disease inflammatory response in the eye.4,5,6 This experimental model is useful for analysing the immunopharmacology of various immunosuppressive agents in uveitis.7
Tacrolimus (FK506) is a substance isolated and purified from metabolites of a fungus, Streptomyces tsukubaensis.8 Tacrolimus binds to and forms a complex with a specific FK506‐binding protein called immunophilin, which is present in abundance in neurones.8,9 This complex inhibits the activation of calcineurin, a Ca2‐dependent enzyme, and exhibits various pharmacological actions.8,9,10 In the field of ophthalmology, tacrolimus was shown to be effective in the treatment of immune‐mediated diseases such as EAU and corneal graft rejection.11,12
To reduce the systemic side effects of orally and intravenously administered drugs, the topical application of a drug is preferable. Recently, sustained‐release corticosteroids delivered by intravitreal implant devices have been effective in the treatment of refractory uveitis.13 However, this approach involves complications such as increased intraocular pressure and cataract.13
We previously showed that intravitreal injection of tacrolimus effectively suppresses ocular inflammation in a rabbit model of experimental uveitis.14 However, the effect of intravitreal injection of tacrolimus for EAU has not been studied. In this study, we investigated whether intravitreal injection of tacrolimus would be effective in the treatment of EAU in Lewis rats.
Materials and methods
Animals
Female Lewis rats, 6–8 weeks‐old, were purchased from Japan CLEA (Shizuoka, Japan). All rats were treated in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. A mixture of ketamine HCl and xylazine was used for anesthesia and administered intraperitoneally by injection.
Reagents
Bovine interphotoreceptor retinoid binding protein peptide (R14)5 was synthesised using Sawady Technology (Tokyo, Japan). Complete Freund's adjuvant was purchased from Difco Labs (Detroit, Michigan, USA). Killed Bordetella pertussis suspension was purchased from Sigma Chemical (St Louis, Missouri, USA).
Induction and scoring of EAU
Lewis rats received an injection into one hind footpad of R14 (0.5 μg) in 0.1 ml emulsion in complete Freund's adjuvant,5 and killed B pertussis suspension (1×1010 cells) were given intraperitoneally as an additional adjuvant.
The eyes were examined daily after R14 immunisation independently by two blinded observers to assess for the onset of inflammation using a slit‐lamp biomicroscope.15 For histological analysis, the animals were killed on day 17 after immunisation. The left eyes were removed and fixed in 10% neutral buffered formalin. Sections were embedded in paraffin wax and stained with haematoxylin and eosin. The severity of ocular inflammation was graded according to the grading system reported previously by Kawashima et al.16
Intravitreal injection of tacrolimus
Tacrolimus (Prograf, Asteras, Tokyo, Japan) was dissolved in balanced saline solution (BSS Plus; Alcon, Fort Worth, Texas, USA) to a concentration of 2 and 1 mg/ml. Tacrolimus solution (5 μl) was injected into the vitreous cavity of rats using a 30‐G needle after paracentesis was performed.
On days 2, 7 and 14 after intravitreal injection, tacrolimus concentrations in the injected eyes and peripheral blood were determined. The tacrolimus concentration was determined by the ELISA systems with a lower detection limit of 0.5 ng/ml.14
Assay of delayed type hypersensitivity
Rats received an intradermal injection of 1 μg/20 μl phosphate‐buffered saline (PBS) of R14 peptide into the left ear pinnae on day 12 after immunisation. After 48 h, the ear swelling was measured by a micrometer (Mitutoyo, MTI, Corporation, Paramus, New Jersey, USA).
Preparation of peritoneal macrophages
Peritoneal macrophages were obtained by lavage from the peritoneum with PBS and resuspended in serum‐free medium composed of RPMI‐1640, 10 mM HEPES, 0.1 mM non‐essential amino acid solution, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Sigma), plated in 96‐well flat bottom plates for 2 h at 37°C with 5% CO2, to allow them to adhere. We placed 2×105 cells/well in a 100 μl culture media, then after 2 h, the plates were washed twice with a culture medium to remove non‐adherent cells. Adherent macrophage monolayers were incubated in complete media composed of RPMI‐1640, 10 mM HEPES, 0.1 mM non‐essential amino acid solution, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum for 24 h. Macrophages were cultured with or without lipopolysacharide (0.1 μg/ml) and in the absence or presence of tacrolimus (0.1 or 1 μg/ml).
Cytokine assay
To assay the supernatant derived from vehicle‐treated or tacrolimus‐treated eyes for content of interferon (IFN)γ, tumour necrosis factor (TNF)α, vehicle‐treated or tacrolimus‐treated eyes were removed on day 14 after immunisation. The eyes were separated into anterior and posterior segments and the lenses were removed. PBS was added, then homogenised, after which they were centrifuged for 15 min at 15 000 rpm. The supernatants were analysed by an ELISA kit (Bioscource, Camarillo, California, USA). In some experiments, the amount of TNFα in the supernatant obtained from cultured macrophages was also measured.
Chemokine gene expression
Total RNA was extracted from the whole retina using an Isogen RNA Isolation Kit (Nippon Gene, Tokyo, Japan). The first‐strand cDNAs were synthesised from 1 μg of total RNA in a 20 μl reaction mixture containing oligo(dT) primer and avian myeloblastosis virus reverse transcriptase, by incubating at 42°C for 60 h. For polymerase chain reaction (PCR) amplification, cDNAs were amplified using primers as shown in table 1. PCR was performed in a 50 μl amplification mixture containing 1× polymerase buffer, 1.5 mM MgCl2, 0.2 mM each of dNTP, 1 μM of forward and reverse primers, and 1.25 U Taq polymerase (Promega, Madison, Wisconsin, USA). PCR cycling conditions were 94°C for 60 s, 55°C for 60 s and 72°C for 60 s, for 30 cycles. The PCR products were separated by electrophoresis in 1% agarose gel containing 0.5 μg/ml ethidium bromide. The bands were analysed by densitometry, and gene expression was expressed as a ratio of the gene of interest to the GAPDH gene.
Table 1 Sequences of rat chemokine and β‐actin primers used in reverse transcription‐polymerase chain reaction.
| Chemokine | Sequences | Product size (bp) |
|---|---|---|
| β‐actin | 5′‐CTGGAGAAGAGCTATGAGCTG‐3′ | 246 |
| 5′‐AATCTCCTTCTGCATCCTGTC‐3′ | ||
| MCP‐1 | 5′‐CTGGGCCTGTTGTTCACAGTTGC‐3′ | 436 |
| 5′‐CTACAGAAGTGCTTGAGGTGGTTG ‐3′ | ||
| RANTES | 5′‐CCATATGGCTCGGACACCA‐3′ | 201 |
| 5′‐GCTCATCTCCAAATAGTT‐3′ | ||
| IL‐8 | 5′‐GAAGATAGATTGCACCGA‐3′ | 365 |
| 5′‐CATAGCCTCTCACACATTTC‐3′ |
IL, interluekin; MCP‐1, monocyte chemoattractant protein‐1; RANTES, regulated on activation, normal T cell expressed and secreted.
Electroretinographic study
Retinal function was evaluated by scotopic electroretinogram (PE200; Tomei, Nagoya, Japan) on days 2 and 7 after intravitreal injection.
Statistical analysis
Results of experiments were analysed using the Mann–Whitney U test and Student's t test. A p value of <0.05 indicated a significant difference.
Results
Toxicity of intravitreal injection of tacrolimus
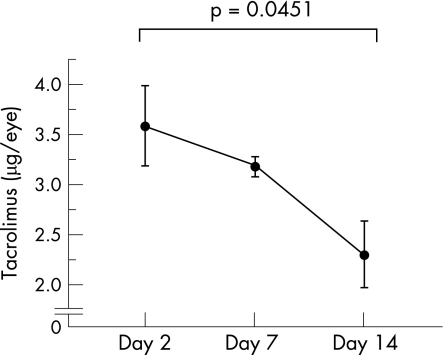
Tacrolimus was injected into the vitreous cavity of non‐immunised rats only once before the electroretinogram examination. From the electroretinogram findings, no changes in waveform and amplitude were observed between tacrolimus‐treated eyes and the untreated normal contralateral eyes. A histopathological examination showed no remarkable abnormalities compared with the control eyes. Serum biochemical tests showed normal renal function (data not shown). The tacrolimus level in the eye was 3.6 ng/eye on day 2, 3.2 ng/eye on day 7 and 2.3 ng/eye on day 14 (fig 1). Mild vitreous opacity was observed in the vitreous of both the tacrolimus‐treated group and the vehicle‐treated group. The reaction seemed to be of the same degree in both groups. The blood concentration was below the detection limit (0.5 ng/ml) throughout the course of observation.
Figure 1 Tacrolimus level in the eye after intravitreal injection in the normal rats. Tacrolimus was injected into the vitreous cavity of non‐immunised rats only once. Tacrolimus concentrations in the supernatants from the eyes that received an intravitreal injection of tacrolimus (10 μg/eye) were measured by ELISA. Data are the mean±SEM (n = 4). The experiment was repeated twice with similar results.
Effect of intravitreal injection of tacrolimus on ocular inflammation in EAU
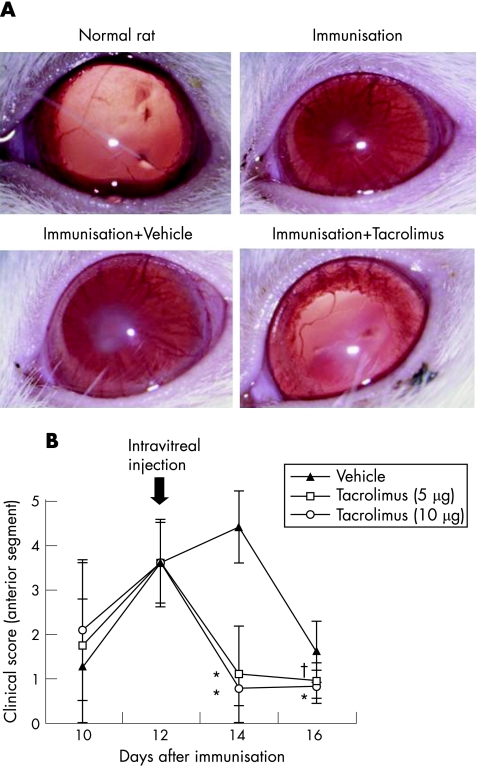
Firstly, we determined whether intravitreal injection of tacrolimus suppresses ocular inflammation of EAU in Lewis rats on the efferent limb. Figure 2A shows the representative results. Both the immunised rats and the immunised rats receiving intravitreal injection of the vehicle showed total synechia and mild fibrin formation at the centre of the pupil. By contrast, the immunised rats receiving intravitreal injection of tacrolimus (10 μg/5 μl/rats) showed only poor mydriasis. As fig 2B shows, the total scores of the anterior segment of the tacrolimus‐treated eyes (5 and 10 μg) were significantly suppressed compared with the vehicle‐treated eyes. These results indicate that intravitreal injection of tacrolimus is effective for the control of ongoing ocular inflammation in the anterior segment of the eye in EAU in rats.
Figure 2 (A) The slit‐lamp biomicroscopic appearance of the eyes of normal rats, immunised rats, immunised rats receiving vehicle and immunised rats receiving tacrolimus on day 14 after immunisation. Intravitreal injection of tacrolimus (10 μg/eye) was performed on day 12 after immunisation. (B) The effect of intravitreal injection of tacrolimus on ocular inflammation in the anterior segment shown by the clinical score. Intravitreal injection of tacrolimus (5 or 10 μg/eye) or vehicles was performed on day 12 after immunisation. Data are the mean±SD (n = 10). *p<0.01 compared with vehicle‐treated eyes. † p<0.05 compared with vehicle‐treated eyes. The experiment was repeated thrice with similar results.
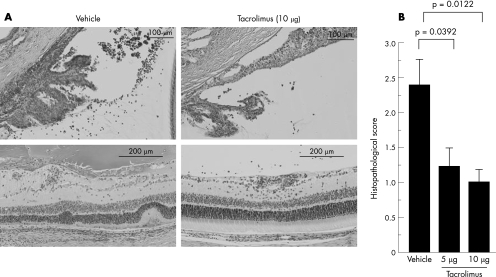
Furthermore, as fig 3A shows, vehicle‐treated eyes showed infiltrating inflammatory cells in the anterior segments and vitreous cavity, retinal vasculitis and damage of the photoreceptor cell layer, whereas tacrolimus‐treated eyes (10 μg) showed only mild inflammation. As fig 3B shows, histopathological scores representing structural damage of the retina were significantly lower in the tacrolimus‐treated groups. These findings suggest that intravitreal injection of tacrolimus is also useful in the treatment of inflammation of the posterior segments of the eyes in EAU in rats.
Figure 3 (A) The effect of intravitreal injection of tacrolimus on histopathological changes (haematoxylin and eosin staining) in the anterior segments (on day 14 after immunisation) and the posterior segments (on day 17 after immunisation) of the vehicle‐treated or tacrolimus‐treated eyes. An intravitreal injection of tacrolimus was performed on day 12 after immunisation. (B) The effect of intravitreal injection of tacrolimus on ocular inflammation on day 17 after immunisation was shown by the histopathological score. Data are the mean±SEM (n = 7). The experiment was repeated thrice with similar results.
Effect of intravitreal injection of tacrolimus on the IFNγ and TNFα protein level in the eye
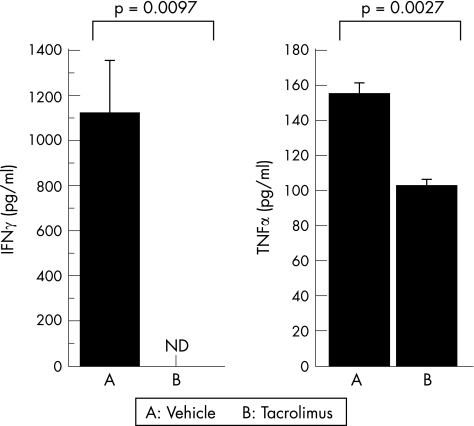
We examined whether intravitreal injection of tacrolimus suppressed the production of inflammatory cytokine, IFNγ and TNFα in the eyes of immunised rats. As fig 4 shows, on day 14, the production of IFNγ and TNFα was markedly suppressed in the tacrolimus‐treated eyes. These results suggest that tacrolimus has the capacity to suppress inflammatory cytokine levels in eyes with ongoing EAU.
Figure 4 The effect of intravitreal injection of tacrolimus on interferon (IFN)γ and tumour necrosis factor (TNF)α levels in the supernatant derived from the eyes of the rats on day 14 after immunisation. Protein levels of IFNγ and TNFα were measured by ELISA. ND, not detected. Data are the means±SEM (n = 3). The experiment was repeated twice with similar results.
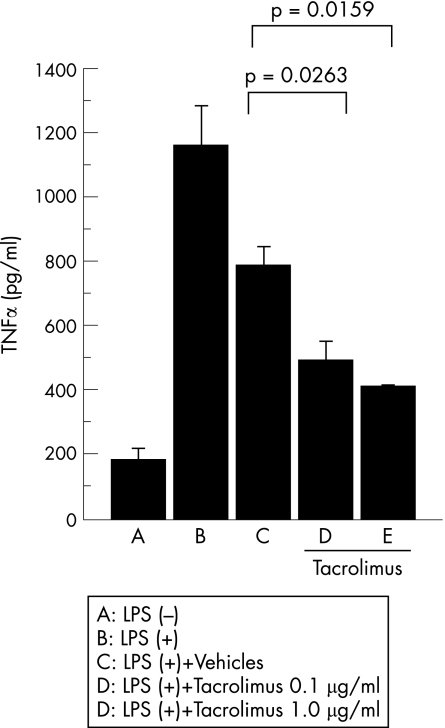
Effect of intravitreal injection of tacrolimus on the TNFα production of the cultured macrophages
Although the immunosuppressive effect of tacrolimus is manifested by inhibiting the nuclear factor of activated T cell to suppress the transcription of interleukin (IL)2,10 recent papers have shown that tacrolimus exhibits an inhibitory effect of inducible nitric oxide synthase expression and nitric oxide production in macrophages.17,18 In addition, both TNFα and IFNγ activate macrophages and elicit strong nitric oxide responses. We therefore investigated whether tacrolimus inhibits TNFα production by cultured rat macrophages in vitro. The amount of TNFα in the supernatant collected from cultured macrophages was measured by ELISA. As fig 5 shows, TNFα production was significantly reduced in the tacrolimus‐treated macrophages. These results indicate that tacrolimus has the capacity to suppress the production of inflammatory cytokines such as TNFα from lipopolysaccharide‐treated macrophages.
Figure 5 The effect of tacrolimus on tumour necrosis factor (TNF)α levels in the supernatant derived from lipopolysaccharide stimulated macrophages. Peritoneal exudates cells treated with vehicle or tacrolimus were with LPS (0.1 μg/ml) for 24 h. The protein level of TNFα was measured by ELISA. Data are the mean±SEM (n = 3). The experiment was repeated twice with similar results.
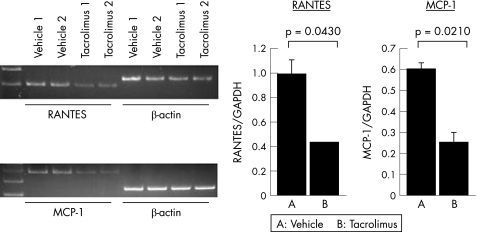
Effects of intravitreal injection of tacrolimus on chemokine mRNA expression in the retina
In the further study, we tested whether intravitreal injection of tacrolimus influence chemokine mRNA expression in the retina of immunised rats. As fig 6 shows, the intravitreal injection of tacrolimus markedly reduced the expression of the monocyte chemoattractant protein‐1 (MCP‐1) and regulated upon activation, normal T cell expressed and secreted (RANTES) mRNA compared with the expression levels in the vehicle‐treated eyes. There was no significant difference in the mRNA of IL8 between the tacrolimus‐treated and the vehicle‐treated eyes (data not shown). Thus, these data suggest that an intravitreal injection of tacrolimus suppresses the mRNA expression of MCP‐1 and RANTES in the eyes with ongoing ocular inflammation.
Figure 6 The effect of the intravitreal injection of tacrolimus on the chemokine mRNA expression in the retina of the rats immunised with R14 on day 14 after immunisation. The intensity of mRNA was analysed, and the relative intensity was determined by a semiquantitative polymerase chain reaction (PCR) and compared with that of β‐actin. Data are the mean(SEM); n = 2. The experiment was repeated twice with similar results.
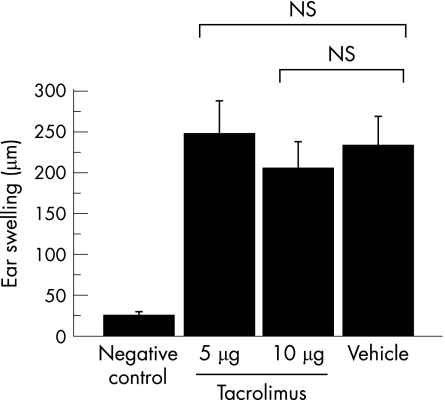
Effects of tacrolimus on delayed‐type hypersensitivity during EAU
Finally, we investigated the effect of an intravitreal injection of tacrolimus on delayed‐type hypersensitivity on the efferent limb of EAU. Measurement of ear swelling response at 48 h showed that both groups of rats that received the intravitreal injection of tacrolimus showed delayed‐type hypersensitivity responses of the intensity comparable to positive controls (fig 7). Thus, the intravitreal injection of tacrolimus does not influence the cell‐mediated systemic immune responses of delayed‐type hypersensitivity.
Figure 7 Effect of the intravitreal injection of tacrolimus on delayed‐type hypersensitivity. An intravitreal injection of tacrolimus (5 or 10 μg/eye) or vehicle was given on day 12 after immunisation. After 30 min, their ear pinnae, as well as pinnae of normal rats (negative control), were challenged with an injection of R14 (1 μg/rat). Ear swelling responses were measured 48 h after the challenge. The bars represent the mean ear swelling responses ±SEM (n = 6). The experiment was repeated twice with similar results.
Discussion
This study clearly showed that intravitreal injection of tacrolimus was capable of suppressing the ongoing process of autoimmune uveoretinitis in the eye, even after the uveitis had been initiated. The above data suggest that the intravitreal injection of tacrolimus should have great advantages for the treatment of patients with uveitis.
Recent papers have shown that intravitreal injection or sustained release of tacrolimus can be effective for experimental uveitis in a rabbit model.14,19 However, the mechanism to suppress ocular inflammation and the effect for systemic immune response remain unknown. In this study, to elucidate the anti‐inflammatory mechanism of the intravitreal injection of tacrolimus, we measured the concentrations of inflammatory cytokines such as IFNγ and TNFα. As shown, both IFNγ and TNFα production were inhibited in the tacrolimus‐treated eyes on day 14 after immunisation. Furthermore, the increasing levels of IFNγ and TNFα corresponded to the clinical score on day 14. On the other hand, delayed‐type hypersensitivity was not suppressed in the tacrolimus‐treated rats, indicating that intravitreal injection of tacrolimus does not influence the cell‐mediated systemic immune response. Taken together, our results indicate that tacrolimus injected into the vitreous cavity has the capacity to suppress effector inflammatory cells via inhibition of the inflammatory cytokine expression in the ocular tissue, not involving the systemic immune responses.
TNFα is a pleotropic cytokine produced principally by activated macrophages and T cells during inflammatory responses.20 In EAU, the increased concentration of TNFα facilitates the ongoing T cell effector function and macrophage activation.20 Therefore, we examined whether tacrolimus influences activated macrophages with regard to the TNFα production in vitro. As we have shown, there was pronounced inhibition of activated macrophage‐derived TNFα in the presence of tacrolimus in a dose‐dependent manner. Bone marrow‐derived macrophages are major effector cells of tissue destruction during EAU.21 Thus, a possible mechanism for the suppression of ongoing EAU by intravitreal injection of tacrolimus is that tacrolimus may affect the activated macrophages producing TNFα in the eye.
It is widely accepted that synthesis and secretion of inflammatory chemokines play an important part in the pathogenesis of ocular inflammation.22,23,24 Our results showed that intravitreal injection of tacrolimus suppressed MCP‐1 and RANTES gene expression in retinas with EAU. Both MCP‐1 and RANTES are potent chemoattractants for T lymphocytes and macrophages, which are infiltrating cells observed in the posterior segment of eyes with EAU.22 This finding may reflect a reduced number of T lymphocytes and macrophages invading the vitreous cavity and retina during the immune response. It is also possible that tacrolimus injected into the vitreous cavity reduces both MCP‐1 and RANTES gene expression on the infiltrating T cells and macrophages. This notion is supported by a previous report showing that both MCP‐1 and RANTES are associated with infiltrating inflammatory cells.22 Our data suggest that inhibition of MCP‐1 and RANTES production by intravitreal injection of tacrolimus may block lymphocyte/macrophage infiltration and subsequent inflammatory reactions in EAU.
In conclusion, intravitreal injection of tacrolimus is an effective local treatment of ongoing EAU and is expected to be clinically applicable for the treatment of human uveitis.
Acknowledgements
We thank JP Baron for reviewing the manuscript.
Abbreviations
EAU - experimental autoimmune uveoretinitis
IFN - interferon
MCP‐1 - monocyte chemoattractant protein‐1
PBS - phosphate‐buffered saline
PCR - polymerase chain reaction
RANTES - regulated upon activation, normal T cell expressed and secreted
TNF - tumour necrosis factor
Footnotes
Funding: This work was supported by Grant‐in‐Aid 17791258 for Scientific Research from the Japan Society for the Promotion of Science.
Competing interests: None.
References
- 1.Caspi R R, Roberge F G, Chan C C.et al A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol 19881401490–1495. [PubMed] [Google Scholar]
- 2.Forrester J V, Liversidge J, Dua H S.et al Comparison of clinical and experimental uveitis. Curr Eye Res 19909(Suppl)75–84. [DOI] [PubMed] [Google Scholar]
- 3.Chan C C, Caspi R R, Ni M.et al Pathology of experimental autoimmune uveoretinitis in mice. J Autoimmun 19903247–255. [DOI] [PubMed] [Google Scholar]
- 4.Gregerson D S, Fling S P, Obritsch W F.et al A new perspective of S‐antigen from immunochemical analysis. Curr Eye Res 19909(Suppl)145–153. [DOI] [PubMed] [Google Scholar]
- 5.Sanui H, Redmond T M, Kotake S.et al Identification of an immunodominant and highly immunopathogenic determinant in the retinal interphotoreceptor retinoid‐binding protein (IRBP). J Exp Med 19891691947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi R R, Silver P B, Chan C C.et al Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol 19961572668–2675. [PubMed] [Google Scholar]
- 7.Forrester J V, Liversidge J, Dua H S.et al Experimental autoimmune uveoretinitis: a model system for immunointervention: a review. Curr Eye Res 199211(Suppl)33–40. [DOI] [PubMed] [Google Scholar]
- 8.Kino T, Hatanaka H, Miyata S.et al FK‐506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK‐506 in vitro. J Antibiot (Tokyo) 1987401256–1265. [DOI] [PubMed] [Google Scholar]
- 9.Kino T, Hatanaka H, Hashimoto M.et al FK‐506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico‐chemical and biological characteristics. J Antibiot (Tokyo) 1987401249–1255. [DOI] [PubMed] [Google Scholar]
- 10.Sawada S, Suzuki G, Kawase Y.et al Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol 19871391797–1803. [PubMed] [Google Scholar]
- 11.Kawashima H, Fujino Y, Mochizuki M. Effects of a new immunosuppressive agent, FK506, on experimental autoimmune uveoretinitis in rats. Invest Ophthalmol Vis Sci 1988291265–1271. [PubMed] [Google Scholar]
- 12.Nishi M, Herbort C P, Matsubara M.et al Effects of the immunosuppressant FK506 on a penetrating keratoplasty rejection model in the rat. Invest Ophthalmol Vis Sci 1993342477–2486. [PubMed] [Google Scholar]
- 13.Jaffe G J, McCallum R M, Branchaud B.et al Long‐term follow‐up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 20051121192–1198. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa T, Hokama H, Katagiri Y.et al Effects of intravitreal injection of tacrolimus (FK506) in experimental uveitis. Curr Eye Res 20053093–101. [DOI] [PubMed] [Google Scholar]
- 15.Okada A A, Keino H, Fukai T.et al Effect of type I interferon on experimental autoimmune uveoretinitis in rats. Ocul Immunol Inflamm 19986215–226. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima H, Mochizuki M. Effects of a new immunosuppressive agent, FK 506, on the efferent limb of the immune responses. Exp Eye Res 199051565–572. [DOI] [PubMed] [Google Scholar]
- 17.Hamalainen M, Lahti A, Moilanen E. Calcineurin inhibitors, cyclosporin A and tacrolimus inhibit expression of inducible nitric oxide synthase in colon epithelial and macrophage cell lines. Eur J Pharmacol 2002448239–244. [DOI] [PubMed] [Google Scholar]
- 18.Strestikova P, Otova B, Filipec M.et al Different mechanisms in inhibition of rat macrophage nitric oxide synthase expression by FK 506 and cyclosporin A. Immunopharmacol Immunotoxicol 20012367–74. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai E, Nozaki M, Okabe K.et al Scleral plug of biodegradable polymers containing tacrolimus (FK506) for experimental uveitis. Invest Ophthalmol Vis Sci 2003444845–4852. [DOI] [PubMed] [Google Scholar]
- 20.Dick A D, Forrester J V, Liversidge J.et al The role of tumour necrosis factor (TNF‐alpha) in experimental autoimmune uveoretinitis (EAU). Prog Retin Eye Res 200423617–637. [DOI] [PubMed] [Google Scholar]
- 21.Forrester J V, Huitinga I, Lumsden L.et al Marrow‐derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr Eye Res 199817426–437. [DOI] [PubMed] [Google Scholar]
- 22.Crane I J, McKillop‐Smith S, Wallace C A.et al Expression of the chemokines MIP‐1alpha, MCP‐1, and RANTES in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci 2001421547–1552. [PubMed] [Google Scholar]
- 23.Foxman E F, Zhang M, Hurst S D.et al Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1‐ versus Th2‐mediated ocular inflammation. J Immunol 20021682483–2492. [DOI] [PubMed] [Google Scholar]
- 24.Keino H, Takeuchi M, Kezuka T.et al Chemokine and chemokine receptor expression during experimental autoimmune uveoretinitis in mice. Graefes Arch Clin Exp Ophthalmol 2003241111–115. [DOI] [PubMed] [Google Scholar]