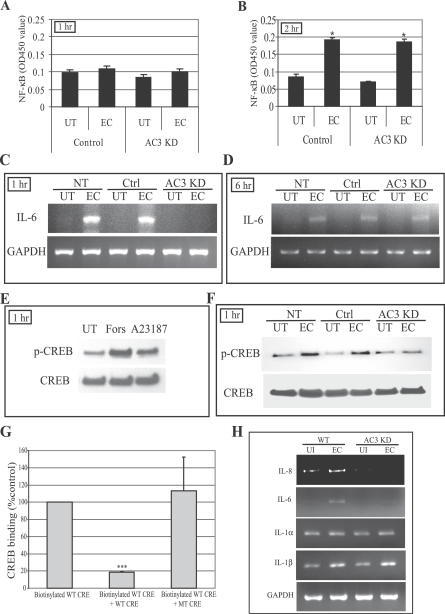
Figure 5. cAMP-Mediated Phosphorylation of CREB and Binding of CREB to CRE Oligonucleotides.
(A and B) NF-κB nuclear translocation in control-transfected BECs and AC-3 KD BECs before and after E. coli (EC) exposure for 1 h (A) or 2 h (B). UT, untreated.
(C and D) IL-6 message levels in non-transfected (NT), control-transfected (Ctrl), and AC-3 KD BECs before and after E. coli exposure for 1 h (C) or 6 h (D) as measured by RT-PCR.
(E) Western blot of CREB phosphorylation levels (p-CREB) in UT BECs, and forskolin (Fors)– or calcium ionophore A23187 (A23187)–treated BECs. The treatment was for 1 h.
(F) Western blot showing CREB phosphorylation of NT, Ctrl, and AC-3 KD BECs before and after 1 h E. coli exposure.
(G) CREB binding to CRE site of the IL-6 promoter. Nuclear extracts of BECs exposed for 1 h to E. coli ORN103(pSH2) were incubated with biotinylated WT CRE oligonucleotides in the absence (Biotinylated WT CRE) or presence of specific (Biotinylated WT CRE + WT CRE) or non-specific (Biotinylated WT CRE + MT CRE) oligonucleotide competitors. ***, p < 0.0001.
(H) Expression analysis of mRNA levels of various genes with CRE sites in their promoter. WT and AC-3 KD BECs were incubated for 1 h with E. coli ORN103(pSH2), and then total RNA was collected from untreated (UI) and bacteria-treated BECs (EC) and subjected to RT-PCR. GAPDH was used as a loading control.