Abstract
The molecular mechanisms responsible for the development of testicular germ cell tumors (GCTs) have not as yet been elucidated. The aim of the present study was to determine whether genetic alterations of p16INK4 (MTS1) and/or cyclin-dependent kinase 4 (CDK4) occur in the genesis of these tumors. We have analyzed these two genes in 29 testicular GCTs, seminomas, and nonseminomas. None of the tumors showed either p16INK4 or CDK4 mutations. Only 1 of the 29 GCTs displayed loss of heterozygosity of the p16INK4 gene. No homozygous deletions of p16INK4 were detected. Evidence of hypermethylation of p16INK4 exon 1, however, was demonstrated in 13 of the 26 (50%) GCTs analyzed. Tumor samples having exon 1 of p16INK4 methylated expressed significantly lower levels of p16INK4 mRNA, as analyzed by reverse transcriptase polymerase chain reaction. These results suggest that p16INK4 inactivation plays a role in the genesis of GCTs.
Full text
PDF

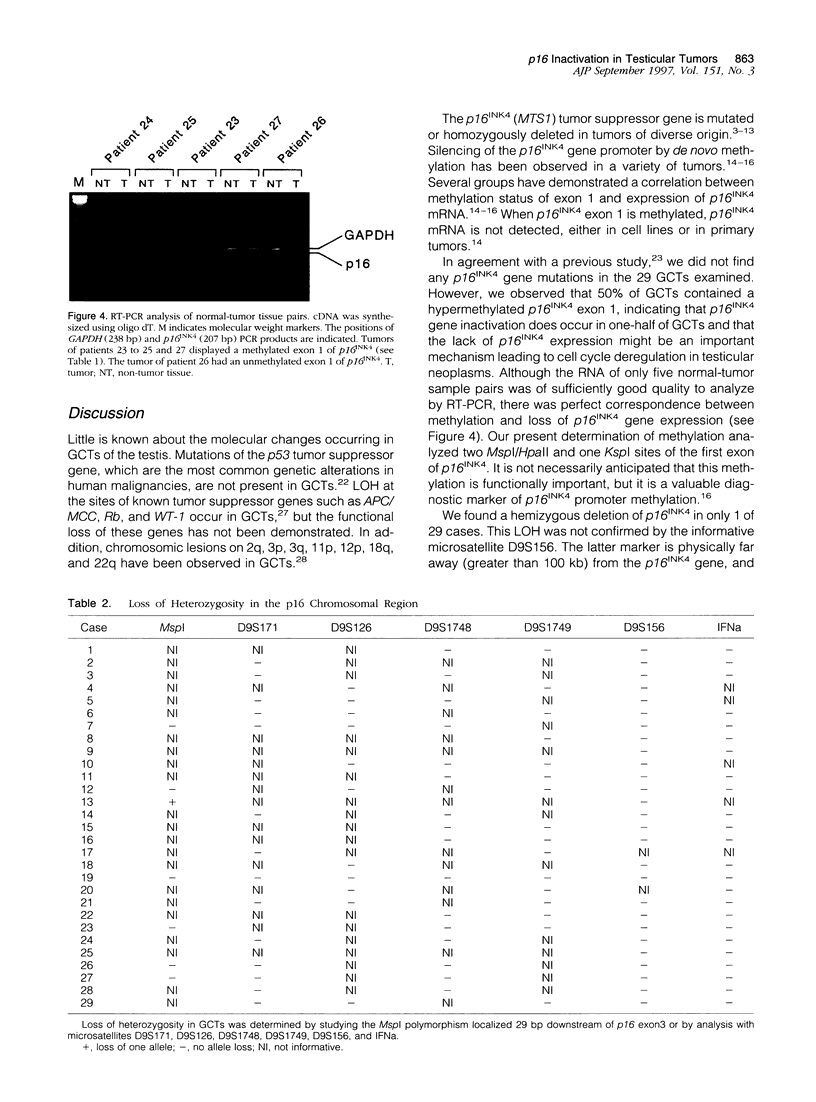


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard L., Lukas J., Bartkova J., Kjerulff A. A., Strauss M., Bartek J. Aberrations of p16Ink4 and retinoblastoma tumour-suppressor genes occur in distinct sub-sets of human cancer cell lines. Int J Cancer. 1995 Mar 29;61(1):115–120. doi: 10.1002/ijc.2910610120. [DOI] [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Chaubert P., Bautista D., Benhattar J. An improved method for rapid screening of DNA mutations by nonradioactive single-strand conformation polymorphism procedure. Biotechniques. 1993 Oct;15(4):586–586. [PubMed] [Google Scholar]
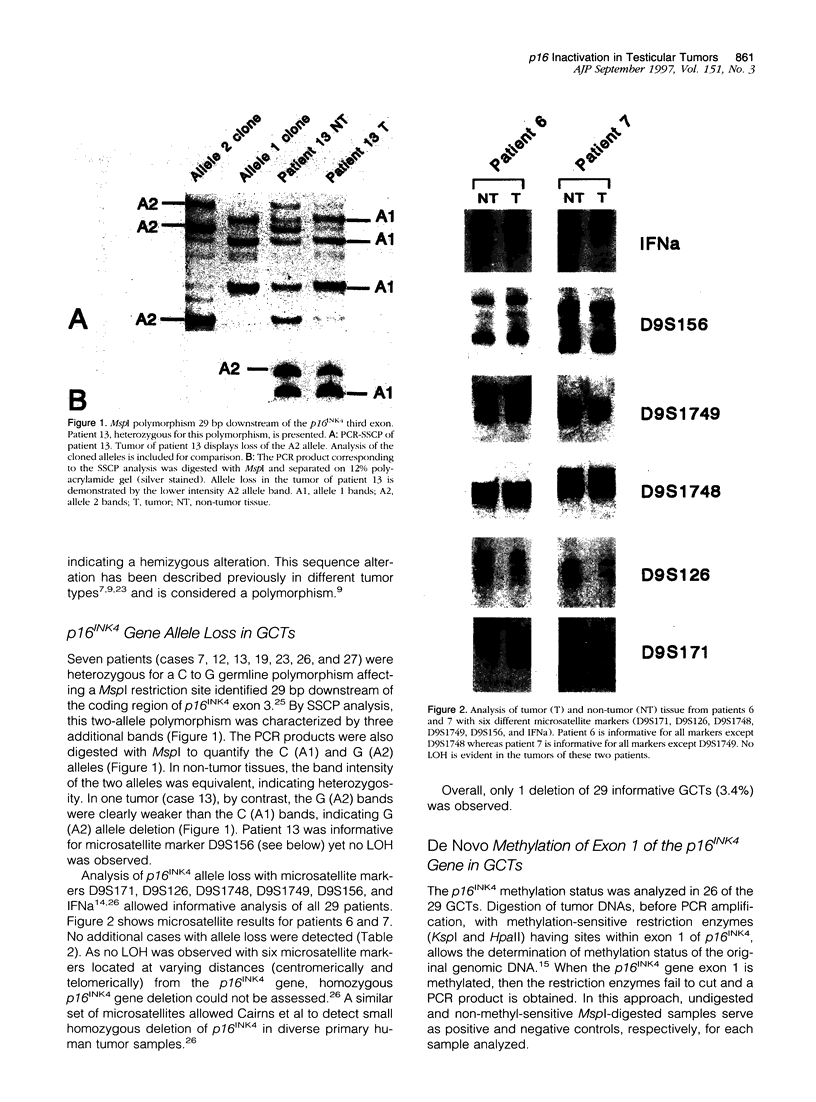
- Chaubert P., Shaw P., Pillet N. Informative MspI polymorphism adjacent to exon 3 of the p16INK4 (MTS1) gene. Mol Cell Probes. 1996 Dec;10(6):467–468. doi: 10.1006/mcpr.1996.0064. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Giani C., Finocchiaro G. Mutation rate of the CDKN2 gene in malignant gliomas. Cancer Res. 1994 Dec 15;54(24):6338–6339. [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Guan K. L., Jenkins C. W., Li Y., Nichols M. A., Wu X., O'Keefe C. L., Matera A. G., Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994 Dec 15;8(24):2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- Guillou L., Estreicher A., Chaubert P., Hurlimann J., Kurt A. M., Metthez G., Iggo R., Gray A. C., Jichlinski P., Leisinger H. J. Germ cell tumors of the testis overexpress wild-type p53. Am J Pathol. 1996 Oct;149(4):1221–1228. [PMC free article] [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Takeuchi S., Lee E., Pham E., Miller C. W., Strohmeyer T., Wilczynski S. P., Melmed S., Koeffler H. P. Alterations of the p16 (MTS1) gene in testicular, ovarian, and endometrial malignancies. J Urol. 1995 Nov;154(5):1954–1957. [PubMed] [Google Scholar]
- Hebert J., Cayuela J. M., Berkeley J., Sigaux F. Candidate tumor-suppressor genes MTS1 (p16INK4A) and MTS2 (p15INK4B) display frequent homozygous deletions in primary cells from T- but not from B-cell lineage acute lymphoblastic leukemias. Blood. 1994 Dec 15;84(12):4038–4044. [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Jen J., Harper J. W., Bigner S. H., Bigner D. D., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994 Dec 15;54(24):6353–6358. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Tommerup N., Fosså S. D., Stenwig A. E., Børresen A. L., Nesland J. M. Molecular genetic changes in human male germ cell tumors. Lab Invest. 1995 Nov;73(5):606–614. [PubMed] [Google Scholar]
- Lukas J., Parry D., Aagaard L., Mann D. J., Bartkova J., Strauss M., Peters G., Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995 Jun 8;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Mao L., Merlo A., Bedi G., Shapiro G. I., Edwards C. D., Rollins B. J., Sidransky D. A novel p16INK4A transcript. Cancer Res. 1995 Jul 15;55(14):2995–2997. [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Mori T., Miura K., Aoki T., Nishihira T., Mori S., Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994 Jul 1;54(13):3396–3397. [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Peng H. Q., Bailey D., Bronson D., Goss P. E., Hogg D. Loss of heterozygosity of tumor suppressor genes in testis cancer. Cancer Res. 1995 Jul 1;55(13):2871–2875. [PubMed] [Google Scholar]
- Peng H. Q., Bailey D., Bronson D., Goss P. E., Hogg D. Loss of heterozygosity of tumor suppressor genes in testis cancer. Cancer Res. 1995 Jul 1;55(13):2871–2875. [PubMed] [Google Scholar]
- Quelle D. E., Zindy F., Ashmun R. A., Sherr C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995 Dec 15;83(6):993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Schmidt E. E., Ichimura K., Reifenberger G., Collins V. P. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res. 1994 Dec 15;54(24):6321–6324. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., DePinho R. A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996 Apr 5;85(1):27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Stone S., Jiang P., Dayananth P., Tavtigian S. V., Katcher H., Parry D., Peters G., Kamb A. Complex structure and regulation of the P16 (MTS1) locus. Cancer Res. 1995 Jul 15;55(14):2988–2994. [PubMed] [Google Scholar]
- Strohmeyer T., Reissmann P., Cordon-Cardo C., Hartmann M., Ackermann R., Slamon D. Correlation between retinoblastoma gene expression and differentiation in human testicular tumors. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6662–6666. doi: 10.1073/pnas.88.15.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel T., Hauer M., Schneider J., Serrano M., Wölfel C., Klehmann-Hieb E., De Plaen E., Hankeln T., Meyer zum Büschenfelde K. H., Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995 Sep 1;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Zhou X., Tarmin L., Yin J., Jiang H. Y., Suzuki H., Rhyu M. G., Abraham J. M., Meltzer S. J. The MTS1 gene is frequently mutated in primary human esophageal tumors. Oncogene. 1994 Dec;9(12):3737–3741. [PubMed] [Google Scholar]