Abstract
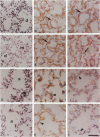
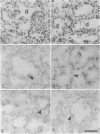
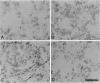
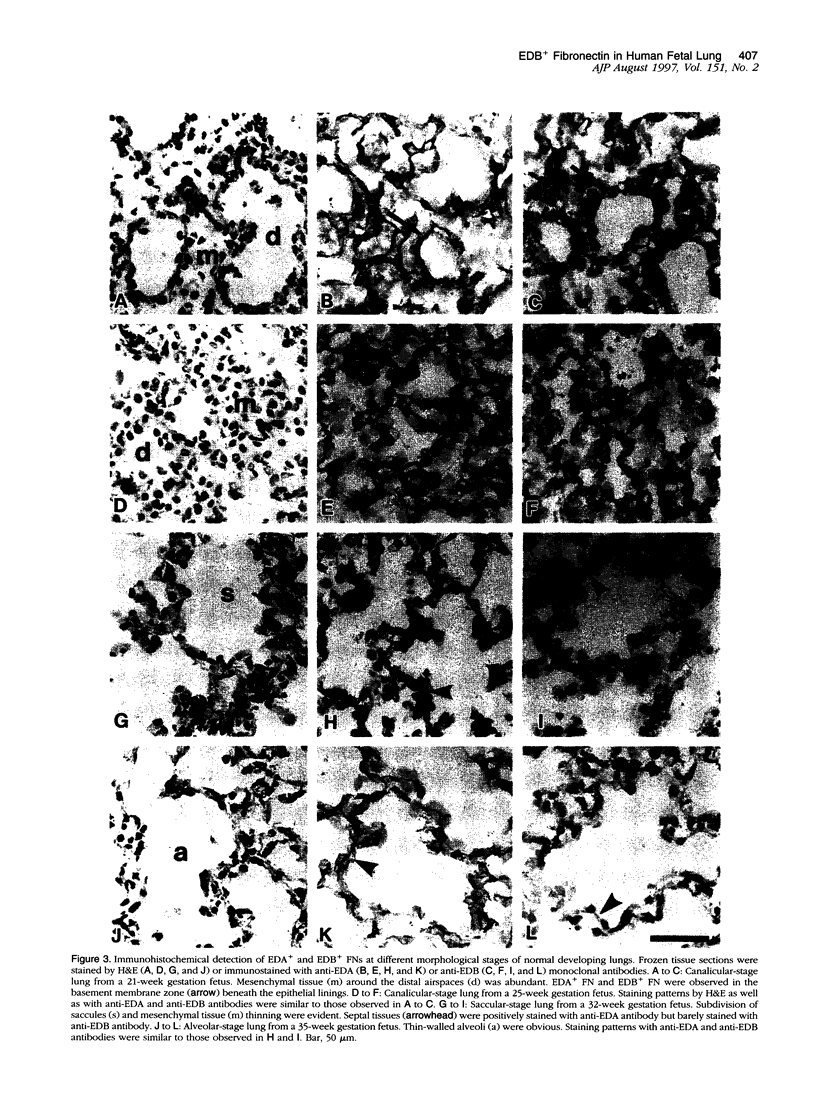
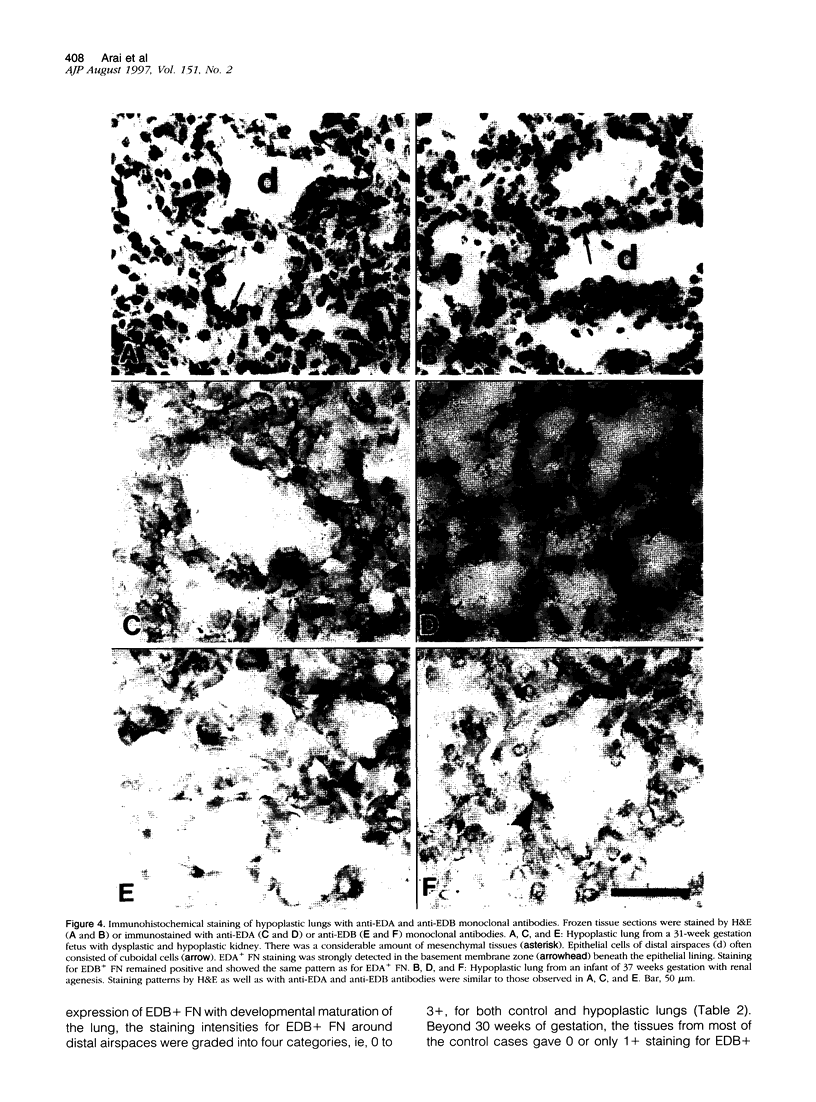
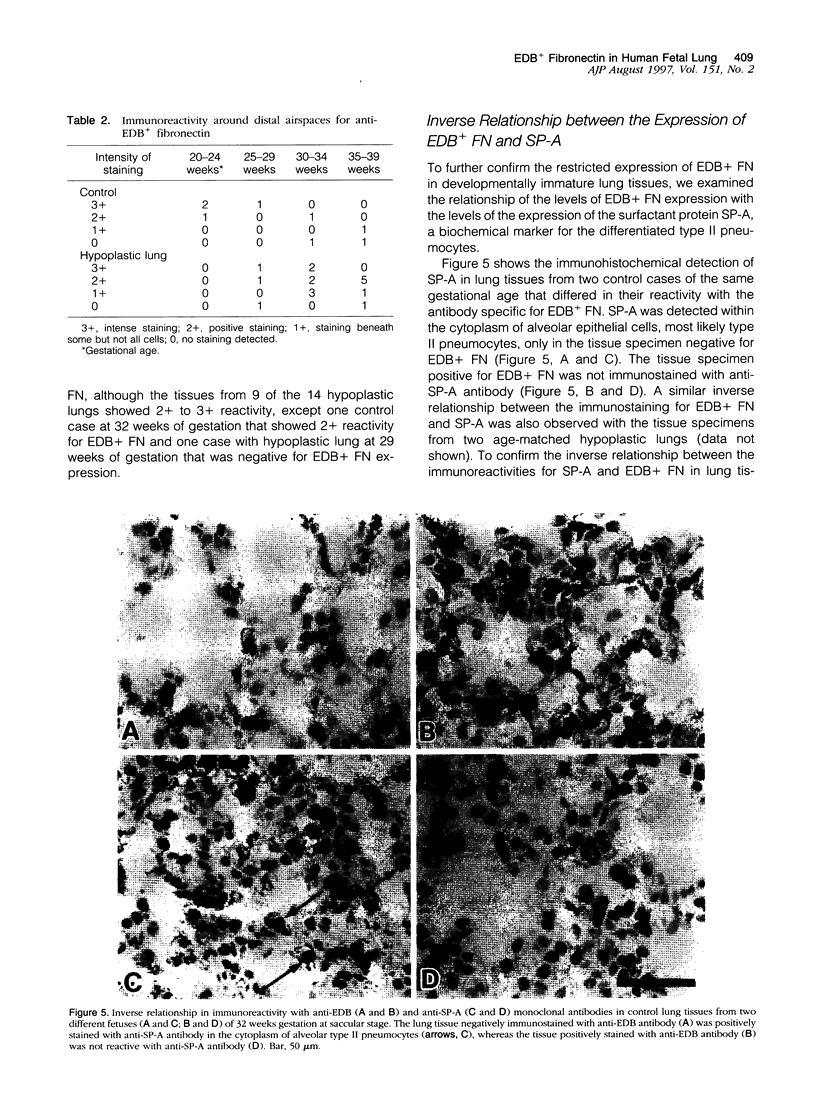
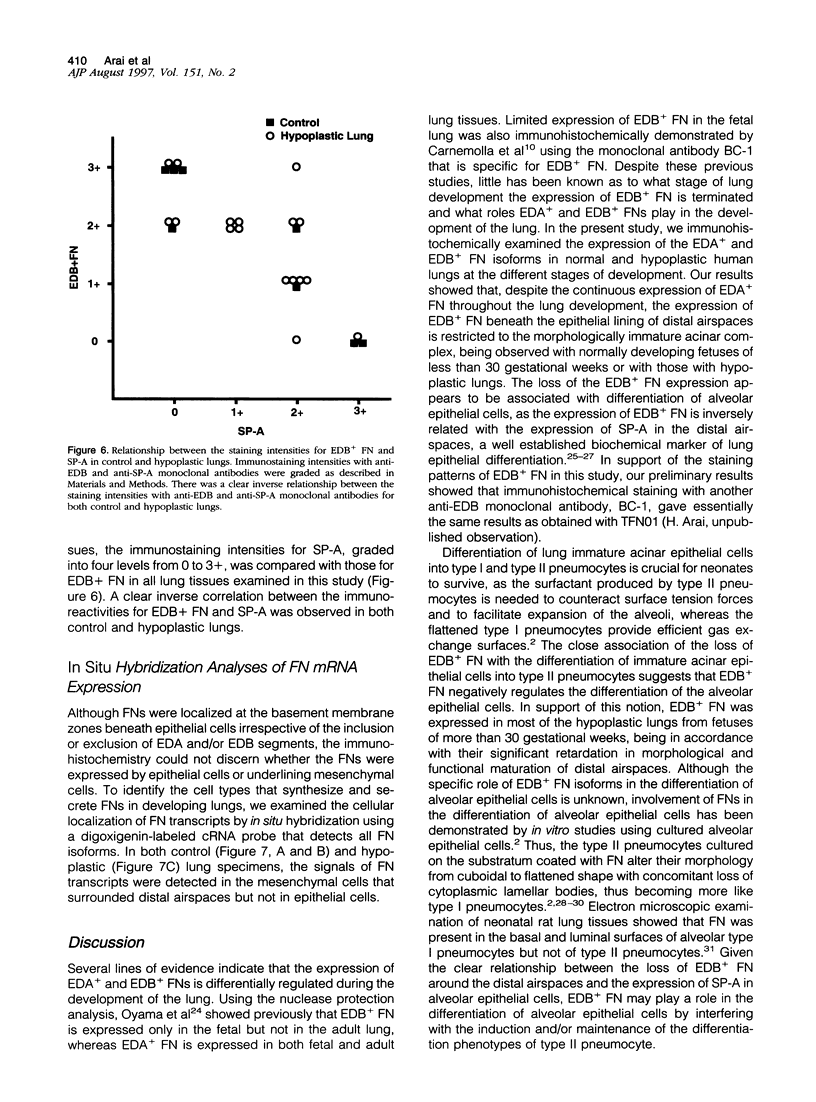
Cell-matrix interactions have been shown to regulate the development of the lung, particularly airway branching and alveolarization. Fibronectin is the major constituent of pulmonary extracellular matrix and exists in multiple isoforms arising from alternative RNA splicing. EDA and EDB are the two major alternatively spliced segments, the expression of which is regulated in a spatiotemporal and oncodevelopmental manner. In this study, we investigated immunohistochemically the distribution of the EDA- and EDB-containing fibronectin isoforms (referred to as EDA+ fibronectin and EDB+ fibronectin, respectively) in normal and hypoplastic human lungs at different gestational ages to explore the role of these fibronectin isoforms in alveolarization. EDA+ fibronectin was expressed around the distal airspaces throughout the development of both normal and hypoplastic lungs. In contrast, the expression of EDB+ fibronectin was restricted to the lung with morphologically immature acinar complex, typically observed in normally developing lungs of < 30 gestational weeks or in hypoplastic lungs. To further confirm the restricted expression of EDB+ fibronectin in immature acinar complex, we examined the correlation of EDB+ fibronectin expression with that of the surfactant protein SP-A, a biochemical marker for the differentiated type II pneumocytes. A clear inverse relationship between the immunoreactivities for EDB+ fibronectin and SP-A was observed in both control and hypoplastic lungs. Given the proposed importance of fibronectins in the differentiation of alveolar epithelial cells, our results suggest that the EDB segment plays a regulatory role in the differentiation of immature acinar epithelial cells into type II pneumocytes. The EDB segment may also serve as a new histochemical marker for the functional maturity of fetal lung tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenazi S. S., Perlman M. Pulmonary hypoplasia: lung weight and radial alveolar count as criteria of diagnosis. Arch Dis Child. 1979 Aug;54(8):614–618. doi: 10.1136/adc.54.8.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström H., Willetts K., Pekny M., Levéen P., Lindahl P., Hedstrand H., Pekna M., Hellström M., Gebre-Medhin S., Schalling M. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996 Jun 14;85(6):863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Brouty-Boyé D., Magnien V. Myofibroblast and concurrent ED-B fibronectin phenotype in human stromal cells cultured from non-malignant and malignant breast tissue. Eur J Cancer. 1994;30A(1):66–73. doi: 10.1016/s0959-8049(05)80021-8. [DOI] [PubMed] [Google Scholar]
- Brouty-Boyé D., Raux H., Azzarone B., Tamboise A., Tamboise E., Béranger S., Magnien V., Pihan I., Zardi L., Israël L. Fetal myofibroblast-like cells isolated from post-radiation fibrosis in human breast cancer. Int J Cancer. 1991 Mar 12;47(5):697–702. doi: 10.1002/ijc.2910470512. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale J. M., Rochat T. R., Moore K. C., Hunninghake G. W. Stereologic analysis of rat type II alveolar cells in vitro. J Lab Clin Med. 1987 Dec;110(6):767–772. [PubMed] [Google Scholar]
- Chen W. T., Chen J. M., Mueller S. C. Coupled expression and colocalization of 140K cell adhesion molecules, fibronectin, and laminin during morphogenesis and cytodifferentiation of chick lung cells. J Cell Biol. 1986 Sep;103(3):1073–1090. doi: 10.1083/jcb.103.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERY J. L., MITHAL A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960 Dec;35:544–547. doi: 10.1136/adc.35.184.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Hallman M., Arjomaa P., Mizumoto M., Akino T. Surfactant proteins in the diagnosis of fetal lung maturity. I. Predictive accuracy of the 35 kD protein, the lecithin/sphingomyelin ratio, and phosphatidylglycerol. Am J Obstet Gynecol. 1988 Mar;158(3 Pt 1):531–535. doi: 10.1016/0002-9378(88)90019-1. [DOI] [PubMed] [Google Scholar]
- Hino K., Shiozawa S., Kuroki Y., Ishikawa H., Shiozawa K., Sekiguchi K., Hirano H., Sakashita E., Miyashita K., Chihara K. EDA-containing fibronectin is synthesized from rheumatoid synovial fibroblast-like cells. Arthritis Rheum. 1995 May;38(5):678–683. doi: 10.1002/art.1780380516. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Pesce C. G., Alonso C. R., Cramer P., Srebrow A., Werbajh S., Muro A. F. The fibronectin gene as a model for splicing and transcription studies. FASEB J. 1996 Feb;10(2):248–257. doi: 10.1096/fasebj.10.2.8641558. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Dempo K., Akino T. Immunohistochemical study of human pulmonary surfactant apoproteins with monoclonal antibodies. Pathologic application for hyaline membrane disease. Am J Pathol. 1986 Jul;124(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- Langston C., Kida K., Reed M., Thurlbeck W. M. Human lung growth in late gestation and in the neonate. Am Rev Respir Dis. 1984 Apr;129(4):607–613. [PubMed] [Google Scholar]
- McGowan S. E. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992 Aug;6(11):2895–2904. [PubMed] [Google Scholar]
- Nakamura Y., Harada K., Yamamoto I., Uemura Y., Okamoto K., Fukuda S., Hashimoto T. Human pulmonary hypoplasia. Statistical, morphological, morphometric, and biochemical study. Arch Pathol Lab Med. 1992 Jun;116(6):635–642. [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Oncodevelopmental regulation of the alternative splicing of fibronectin pre-messenger RNA in human lung tissues. Cancer Res. 1990 Feb 15;50(4):1075–1078. [PubMed] [Google Scholar]
- Pagani F., Zagato L., Vergani C., Casari G., Sidoli A., Baralle F. E. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol. 1991 Jun;113(5):1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P., Hammann A., Moutet M., Samuel J. L., Martin F., Martin M. Expression of fibronectin ED-A+ and ED-B+ isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am J Pathol. 1996 Feb;148(2):579–592. [PMC free article] [PubMed] [Google Scholar]
- Rannels S. R., Fisher C. S., Heuser L. J., Rannels D. E. Culture of type II pneumocytes on a type II cell-derived fibronectin-rich matrix. Am J Physiol. 1987 Dec;253(6 Pt 1):C759–C765. doi: 10.1152/ajpcell.1987.253.6.C759. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Rannels D. E. The type II pneumocyte as a model of lung cell interaction with the extracellular matrix. J Mol Cell Cardiol. 1989 Feb;21 (Suppl 1):151–159. doi: 10.1016/0022-2828(89)90851-1. [DOI] [PubMed] [Google Scholar]
- Roman J., Crouch E. C., McDonald J. A. Reagents that inhibit fibronectin matrix assembly of cultured cells also inhibit lung branching morphogenesis in vitro. Implications for lung development, injury, and repair. Chest. 1991 Mar;99(3 Suppl):20S–21S. [PubMed] [Google Scholar]
- Roman J., McDonald J. A. Expression of fibronectin, the integrin alpha 5, and alpha-smooth muscle actin in heart and lung development. Am J Respir Cell Mol Biol. 1992 May;6(5):472–480. doi: 10.1165/ajrcmb/6.5.472. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Young S. L., Mendelson C. R. Molecular and cellular processing of lung surfactant. FASEB J. 1994 Sep;8(12):957–967. doi: 10.1096/fasebj.8.12.8088461. [DOI] [PubMed] [Google Scholar]
- Rosenkrans W. A., Jr, Albright J. T., Hausman R. E., Penney D. P. Ultrastructural immunocytochemical localization of fibronectin in the developing rat lung. Cell Tissue Res. 1983;234(1):165–177. doi: 10.1007/BF00217410. [DOI] [PubMed] [Google Scholar]
- Schuger L., O'Shea S., Rheinheimer J., Varani J. Laminin in lung development: effects of anti-laminin antibody in murine lung morphogenesis. Dev Biol. 1990 Jan;137(1):26–32. doi: 10.1016/0012-1606(90)90004-3. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Klos A. M., Hirohashi S., Hakomori S. Human tissue fibronectin: expression of different isotypes in the adult and fetal tissues. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1012–1017. doi: 10.1016/s0006-291x(86)80145-0. [DOI] [PubMed] [Google Scholar]
- Sinkin R. A., Sanders R. S., Horowitz S., Finkelstein J. N., Lomonaco M. B. Cell-specific expression of fibronectin in adult and developing rabbit lung. Pediatr Res. 1995 Feb;37(2):189–195. doi: 10.1203/00006450-199502000-00011. [DOI] [PubMed] [Google Scholar]
- Snyder J. M., O'Brien J. A., Rodgers H. F. Localization and accumulation of fibronectin in rabbit fetal lung tissue. Differentiation. 1987;34(1):32–39. doi: 10.1111/j.1432-0436.1987.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Thurlbeck W. M. Prematurity and the developing lung. Clin Perinatol. 1992 Sep;19(3):497–519. [PubMed] [Google Scholar]
- Zardi L., Carnemolla B., Siri A., Petersen T. E., Paolella G., Sebastio G., Baralle F. E. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987 Aug;6(8):2337–2342. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant C. Alternative splicing of fibronectin--many different proteins but few different functions. Exp Cell Res. 1995 Dec;221(2):261–271. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]