Abstract
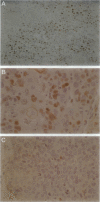
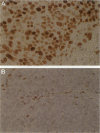
Human papillomavirus (HPV) DNA has been detected in approximately 15% of squamous cell carcinomas (SCCs) of the head and neck. Recent studies have shown a predilection of HPV for certain anatomical sites, especially the tonsillar region, with viral DNA identified in approximately 60% of SCCs of the Waldeyer's tonsillar ring. This study was undertaken to determine whether there are differences in morphology or in oncogene expression in SCC of the tonsil with and without detectable HPV DNA. Twenty-two SCCs of the tonsil were analyzed for the presence of HPV DNA by polymerase chain reaction (PCR) using both a consensus primer set (My09/My11) and type-specific primers. Viral transcription was established in both primary and metastatic tumors by RNA in situ hybridization. The morphology of invasive SCC was classified into three subtypes: well keratinized (K-SCC), intermediate keratinized (I-SCC), and poorly keratinized (P-SCC). Expression of p53, pRB, and cyclin D1 (bcl-1) were studied by immunohistochemistry. In these cases (6 K-SCCs, 2 I-SCCs, and 14 P-SCCs), HPV DNA was detected in 14 (64%), with 11 containing HPV-16 (10 P-SCCs, 1 I-SCCs, and 0 K-SCCs) and 1 each containing HPV-33, HPV-59, and an unclassified HPV type (all P-SCCs). Viral oncoprotein E6/E7 transcription was demonstrated in 7 of 7 HPV-16-positive tumors. Cyclin D1 protein overexpression was detected in the majority of HPV-negative tumors (7 of 8 cases), whereas it was minimal or absent in 13 HPV-positive tumors. Overexpression of p53 protein was detected in 3 HPV-negative K-SCCs. In the HPV-positive tumors, fewer malignant cells expressed pRB and the staining was less intense than in the HPV-negative cancers. HPV DNA and E6/E7 expression, especially HPV-16, is detected in the majority of tonsillar SCCs and is almost exclusively associated with a poorly keratinized tumor histology. Decreased expression of cyclin D1, pRB, and p53 in tumors with HPV DNA is consistent with the known effects of the viral oncoproteins on the cellular protein. The morphology of the HPV-positive tumors suggests that HPV may have a predilection for a population of nonkeratinizing squamous cells or that the virally transformed cells inhibit keratinization of the tumor cells. Well keratinized tonsillar SCCs lack HPV DNA and are associated with overexpression of cyclin D1 protein and/or p53, suggesting that mechanisms that alter the cell cycle regulatory proteins, either by interaction with viral oncoproteins or by changes in the cellular proteins themselves, is critical for tumorigenesis of tonsillar SCC.
Full text
PDF


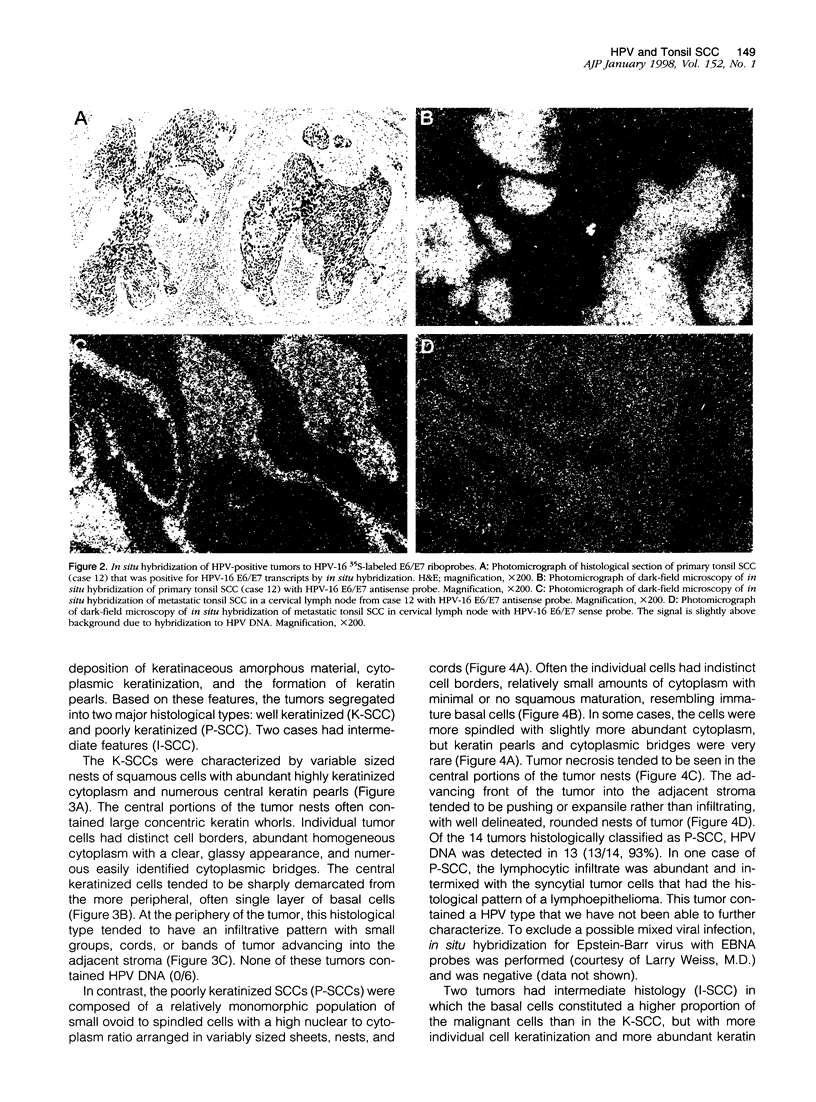

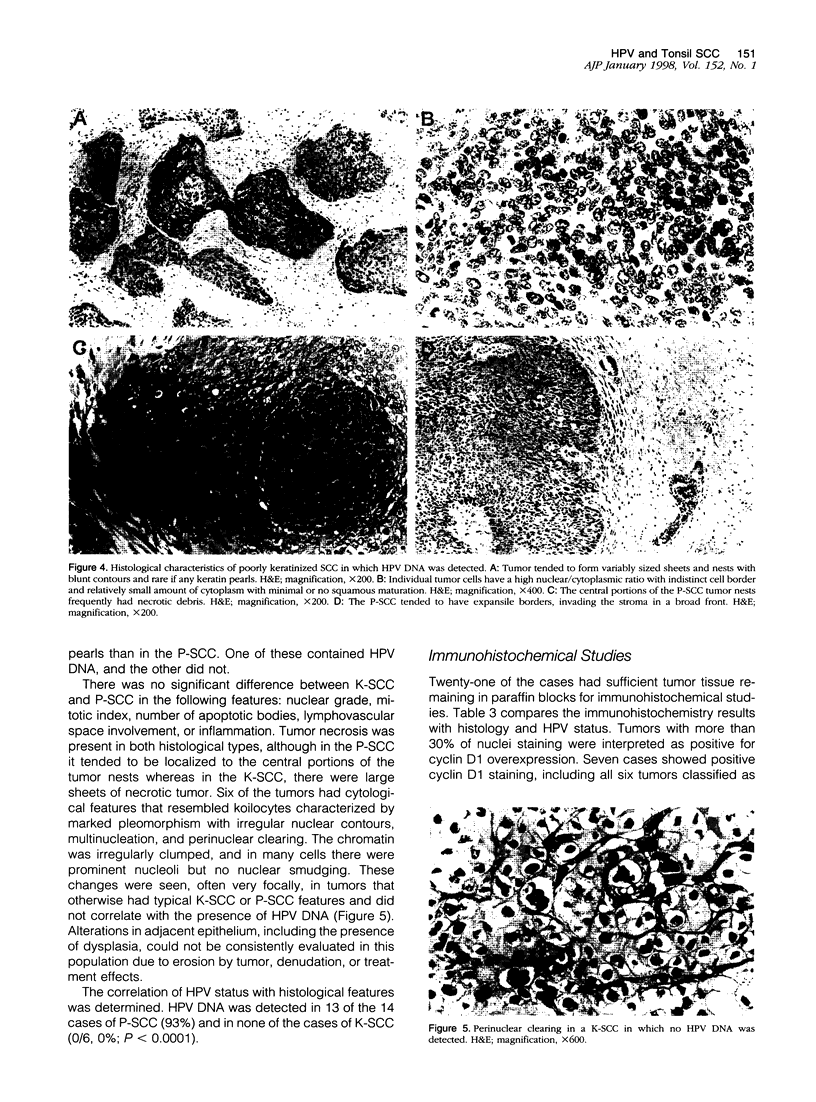
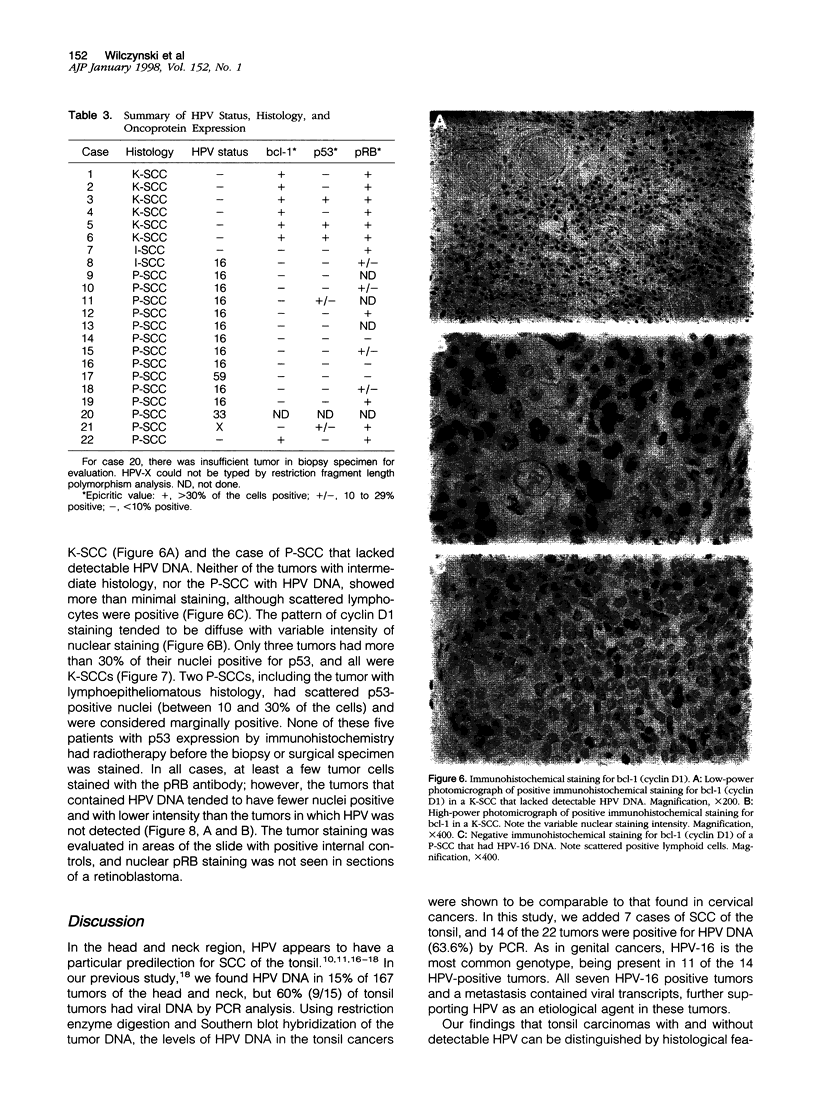
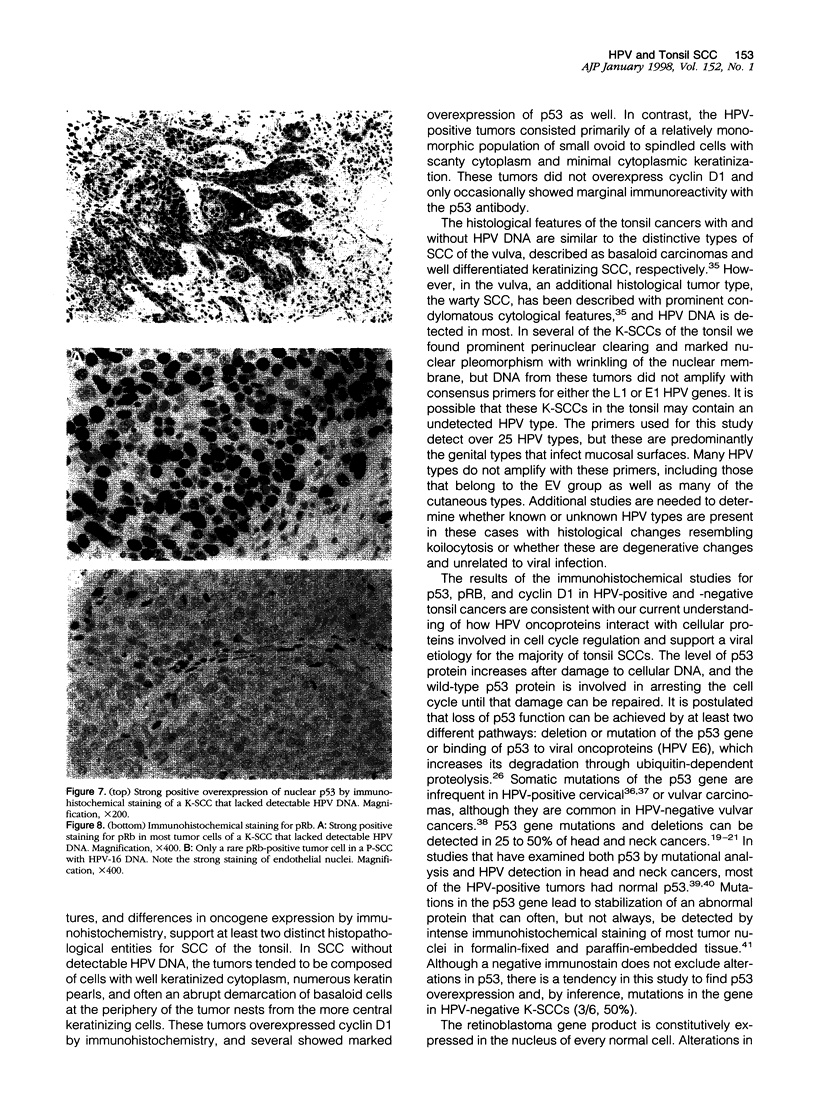



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloss J. D., Liao S. Y., Wilczynski S. P., Macri C., Walker J., Peake M., Berman M. L. Clinical and histologic features of vulvar carcinomas analyzed for human papillomavirus status: evidence that squamous cell carcinoma of the vulva has more than one etiology. Hum Pathol. 1991 Jul;22(7):711–718. doi: 10.1016/0046-8177(91)90294-y. [DOI] [PubMed] [Google Scholar]
- Boyer S. N., Wazer D. E., Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996 Oct 15;56(20):4620–4624. [PubMed] [Google Scholar]
- Brachman D. G., Graves D., Vokes E., Beckett M., Haraf D., Montag A., Dunphy E., Mick R., Yandell D., Weichselbaum R. R. Occurrence of p53 gene deletions and human papilloma virus infection in human head and neck cancer. Cancer Res. 1992 Sep 1;52(17):4832–4836. [PubMed] [Google Scholar]
- Brennan J. A., Boyle J. O., Koch W. M., Goodman S. N., Hruban R. H., Eby Y. J., Couch M. J., Forastiere A. A., Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995 Mar 16;332(11):712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- Chan S. Y., Delius H., Halpern A. L., Bernard H. U. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny, and taxonomy. J Virol. 1995 May;69(5):3074–3083. doi: 10.1128/jvi.69.5.3074-3083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Syrjänen S., Shen Q., Ji H. X., Syrjänen K. Human papillomavirus (HPV) DNA in esophageal precancer lesions and squamous cell carcinomas from China. Int J Cancer. 1990 Jan 15;45(1):21–25. doi: 10.1002/ijc.2910450106. [DOI] [PubMed] [Google Scholar]
- Chen B., Yin H., Dhurandhar N. Detection of human papillomavirus DNA in esophageal squamous cell carcinomas by the polymerase chain reaction using general consensus primers. Hum Pathol. 1994 Sep;25(9):920–923. doi: 10.1016/0046-8177(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Choo K. B., Chong K. Y. Absence of mutation in the p53 and the retinoblastoma susceptibility genes in primary cervical carcinomas. Virology. 1993 Apr;193(2):1042–1046. doi: 10.1006/viro.1993.1224. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Wartinger D., Petrylak D., Dalbagni G., Fair W. R., Fuks Z., Reuter V. E. Altered expression of the retinoblastoma gene product: prognostic indicator in bladder cancer. J Natl Cancer Inst. 1992 Aug 19;84(16):1251–1256. doi: 10.1093/jnci/84.16.1251. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Favre A., Paoli D., Poletti M., Marzoli A., Pesce G., Giampalmo A., Rossi F. The human palatine tonsil studied from surgical specimens at all ages and in various pathological conditions. 1. Morphological and structural analyses. Z Mikrosk Anat Forsch. 1986;100(1):7–33. [PubMed] [Google Scholar]
- Franceschi S., Muñoz N., Bosch X. F., Snijders P. J., Walboomers J. M. Human papillomavirus and cancers of the upper aerodigestive tract: a review of epidemiological and experimental evidence. Cancer Epidemiol Biomarkers Prev. 1996 Jul;5(7):567–575. [PubMed] [Google Scholar]
- Gregoire L., Arella M., Campione-Piccardo J., Lancaster W. D. Amplification of human papillomavirus DNA sequences by using conserved primers. J Clin Microbiol. 1989 Dec;27(12):2660–2665. doi: 10.1128/jcm.27.12.2660-2665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraf D. J., Nodzenski E., Brachman D., Mick R., Montag A., Graves D., Vokes E. E., Weichselbaum R. R. Human papilloma virus and p53 in head and neck cancer: clinical correlates and survival. Clin Cancer Res. 1996 Apr;2(4):755–762. [PubMed] [Google Scholar]
- Jablonska S., Majewski S. Epidermodysplasia verruciformis: immunological and clinical aspects. Curr Top Microbiol Immunol. 1994;186:157–175. doi: 10.1007/978-3-642-78487-3_9. [DOI] [PubMed] [Google Scholar]
- Kotelnikov V. M., Coon J. S., 4th, Mundle S., Kelanic S., LaFollette S., Taylor S I. V., Hutchinson J., Panje W., Caldarelli D. D., Preisler H. D. Cyclin D1 expression in squamous cell carcinomas of the head and neck and in oral mucosa in relation to proliferation and apoptosis. Clin Cancer Res. 1997 Jan;3(1):95–101. [PubMed] [Google Scholar]
- Kurman R. J., Toki T., Schiffman M. H. Basaloid and warty carcinomas of the vulva. Distinctive types of squamous cell carcinoma frequently associated with human papillomaviruses. Am J Surg Pathol. 1993 Feb;17(2):133–145. doi: 10.1097/00000478-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Lee Y. Y., Wilczynski S. P., Chumakov A., Chih D., Koeffler H. P. Carcinoma of the vulva: HPV and p53 mutations. Oncogene. 1994 Jun;9(6):1655–1659. [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Li Y., Nichols M. A., Shay J. W., Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994 Dec 1;54(23):6078–6082. [PubMed] [Google Scholar]
- Lukas J., Pagano M., Staskova Z., Draetta G., Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumour cell lines. Oncogene. 1994 Mar;9(3):707–718. [PubMed] [Google Scholar]
- Michalides R., van Veelen N., Hart A., Loftus B., Wientjens E., Balm A. Overexpression of cyclin D1 correlates with recurrence in a group of forty-seven operable squamous cell carcinomas of the head and neck. Cancer Res. 1995 Mar 1;55(5):975–978. [PubMed] [Google Scholar]
- Monk B. J., Cook N., Ahn C., Vasilev S. A., Berman M. L., Wilczynski S. P. Comparison of the polymerase chain reaction and Southern blot analysis in detecting and typing human papilloma virus deoxyribonucleic acid in tumors of the lower female genital tract. Diagn Mol Pathol. 1994 Dec;3(4):283–291. doi: 10.1097/00019606-199412000-00012. [DOI] [PubMed] [Google Scholar]
- Müller H., Lukas J., Schneider A., Warthoe P., Bartek J., Eilers M., Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols G. E., Williams M. E., Gaffey M. J., Stoler M. H. Cyclin D1 gene expression in human cervical neoplasia. Mod Pathol. 1996 Apr;9(4):418–425. [PubMed] [Google Scholar]
- Niedobitek G., Pitteroff S., Herbst H., Shepherd P., Finn T., Anagnostopoulos I., Stein H. Detection of human papillomavirus type 16 DNA in carcinomas of the palatine tonsil. J Clin Pathol. 1990 Nov;43(11):918–921. doi: 10.1136/jcp.43.11.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Jarzabek-Chorzelska M., Obalek S., Rzesa G., Favre M., Croissant O. Characteristics of the lesions and risk of malignant conversion associated with the type of human papillomavirus involved in epidermodysplasia verruciformis. Cancer Res. 1979 Mar;39(3):1074–1082. [PubMed] [Google Scholar]
- Paquette R. L., Lee Y. Y., Wilczynski S. P., Karmakar A., Kizaki M., Miller C. W., Koeffler H. P. Mutations of p53 and human papillomavirus infection in cervical carcinoma. Cancer. 1993 Aug 15;72(4):1272–1280. doi: 10.1002/1097-0142(19930815)72:4<1272::aid-cncr2820720420>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Paz I. B., Cook N., Odom-Maryon T., Xie Y., Wilczynski S. P. Human papillomavirus (HPV) in head and neck cancer. An association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer. 1997 Feb 1;79(3):595–604. doi: 10.1002/(sici)1097-0142(19970201)79:3<595::aid-cncr24>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Peters G. The D-type cyclins and their role in tumorigenesis. J Cell Sci Suppl. 1994;18:89–96. doi: 10.1242/jcs.1994.supplement_18.13. [DOI] [PubMed] [Google Scholar]
- Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987;48:113–147. doi: 10.1016/s0065-230x(08)60691-0. [DOI] [PubMed] [Google Scholar]
- Resnick R. M., Cornelissen M. T., Wright D. K., Eichinger G. H., Fox H. S., ter Schegget J., Manos M. M. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990 Sep 19;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- Rugge M., Bovo D., Busatto G., Parenti A. R., Fawzy S., Guido M., Ancona E., Ninfo V., Ruol A., Shiao Y. H. p53 alterations but no human papillomavirus infection in preinvasive and advanced squamous esophageal cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1997 Mar;6(3):171–176. [PubMed] [Google Scholar]
- Selivanova G., Wiman K. G. p53: a cell cycle regulator activated by DNA damage. Adv Cancer Res. 1995;66:143–180. doi: 10.1016/s0065-230x(08)60253-5. [DOI] [PubMed] [Google Scholar]
- Shin D. M., Hittelman W. N., Hong W. K. Biomarkers in upper aerodigestive tract tumorigenesis: a review. Cancer Epidemiol Biomarkers Prev. 1994 Dec;3(8):697–709. [PubMed] [Google Scholar]
- Sidransky D. Molecular genetics of head and neck cancer. Curr Opin Oncol. 1995 May;7(3):229–233. doi: 10.1097/00001622-199505000-00007. [DOI] [PubMed] [Google Scholar]
- Slípka J. Palatine tonsils--their evolution and ontogeny. Acta Otolaryngol Suppl. 1988;454:18–22. [PubMed] [Google Scholar]
- Snijders P. J., Cromme F. V., van den Brule A. J., Schrijnemakers H. F., Snow G. B., Meijer C. J., Walboomers J. M. Prevalence and expression of human papillomavirus in tonsillar carcinomas, indicating a possible viral etiology. Int J Cancer. 1992 Jul 30;51(6):845–850. doi: 10.1002/ijc.2910510602. [DOI] [PubMed] [Google Scholar]
- Snijders P. J., van den Brule A. J., Meijer C. J., Walboomers J. M. Papillomaviruses and cancer of the upper digestive and respiratory tracts. Curr Top Microbiol Immunol. 1994;186:177–198. doi: 10.1007/978-3-642-78487-3_10. [DOI] [PubMed] [Google Scholar]
- Soong R., Robbins P. D., Dix B. R., Grieu F., Lim B., Knowles S., Williams K. E., Turbett G. R., House A. K., Iacopetta B. J. Concordance between p53 protein overexpression and gene mutation in a large series of common human carcinomas. Hum Pathol. 1996 Oct;27(10):1050–1055. doi: 10.1016/s0046-8177(96)90282-8. [DOI] [PubMed] [Google Scholar]
- Strohmeyer T., Reissmann P., Cordon-Cardo C., Hartmann M., Ackermann R., Slamon D. Correlation between retinoblastoma gene expression and differentiation in human testicular tumors. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6662–6666. doi: 10.1073/pnas.88.15.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornesello M. L., Buonaguro F. M., Beth-Giraldo E., Kyalwazi S. K., Giraldo G. Human papillomavirus (HPV) DNA in penile carcinomas and in two cell lines from high-incidence areas for genital cancers in Africa. Int J Cancer. 1992 Jun 19;51(4):587–592. doi: 10.1002/ijc.2910510414. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Shen L. H., Crum C. P., Dean P. J., Odze R. D. Low prevalence of human papillomavirus infection in esophageal squamous cell carcinomas from North America: analysis by a highly sensitive and specific polymerase chain reaction-based approach. Hum Pathol. 1997 Feb;28(2):174–178. doi: 10.1016/s0046-8177(97)90102-7. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Wilczynski S. P., Oft M., Cook N., Liao S. Y., Iftner T. Human papillomavirus type 6 in squamous cell carcinoma of the bladder and cervix. Hum Pathol. 1993 Jan;24(1):96–102. doi: 10.1016/0046-8177(93)90068-r. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Kuppuswamy D., Li Y., Livanos E. M., Hixon M., White A., Beach D., Tlsty T. D. Alteration of cell cycle kinase complexes in human papillomavirus E6- and E7-expressing fibroblasts precedes neoplastic transformation. J Virol. 1996 Feb;70(2):999–1008. doi: 10.1128/jvi.70.2.999-1008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Cagle P. T., Moore G. E., Benedict W. F. Absence of retinoblastoma protein expression in primary non-small cell lung carcinomas. Cancer Res. 1991 May 15;51(10):2735–2739. [PubMed] [Google Scholar]
- Zaki S. R., Judd R., Coffield L. M., Greer P., Rolston F., Evatt B. L. Human papillomavirus infection and anal carcinoma. Retrospective analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1992 Jun;140(6):1345–1355. [PMC free article] [PubMed] [Google Scholar]
- Zerfass-Thome K., Zwerschke W., Mannhardt B., Tindle R., Botz J. W., Jansen-Dürr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene. 1996 Dec 5;13(11):2323–2330. [PubMed] [Google Scholar]
- Zerfass K., Schulze A., Spitkovsky D., Friedman V., Henglein B., Jansen-Dürr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995 Oct;69(10):6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Human pathogenic papillomavirus types: an update. Curr Top Microbiol Immunol. 1994;186:1–12. doi: 10.1007/978-3-642-78487-3_1. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., de Villiers E. M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]