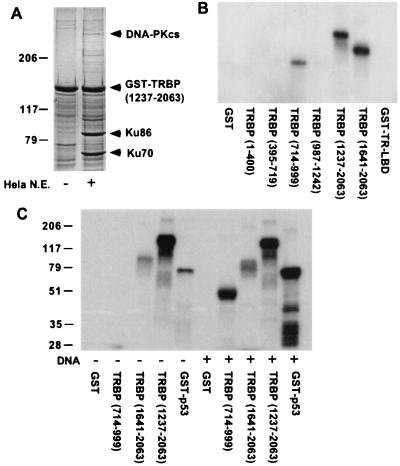
Figure 4.
DNA-PK complex interacts with TRBP and phosphorylates TRBP C terminus in the absence of DNA. (A) HeLa nuclear extract was incubated with GST- TRBP-(1237–2063), and bound nuclear proteins were analyzed by SDS/PAGE with Coomassie blue staining. Protein bands of interest were excised from the gel and subjected to protein microsequencing. DNA-PK catalytic subunit (cs) and regulatory subunits, Ku70 and Ku86, identified by protein sequencing are indicated. (B) DNA-PK phosphorylates TRBP in vitro. GST-TRBP fusion proteins indicated were used as substrates in DNA-PK kinase assay in the presence of linear double-stranded DNA as DNA-PK activator. Phosphorylated TRBP fusion proteins ([γ-32P]ATP labeled) were detected by autoradiography. GST alone and GST-TR-LBD were used as negative controls. (C) DNA-PK phosphorylation of TRBP C terminus is DNA independent. DNA-PK kinase assay was performed with (+) and without (−) linear double-stranded DNA by using TRBP C-terminal (residues 1237–2063 and 1641–2063) or TRBP middle region (residues 714–999) GST fusion proteins as substrates. GST alone and GST-p53 are negative and positive controls, respectively.