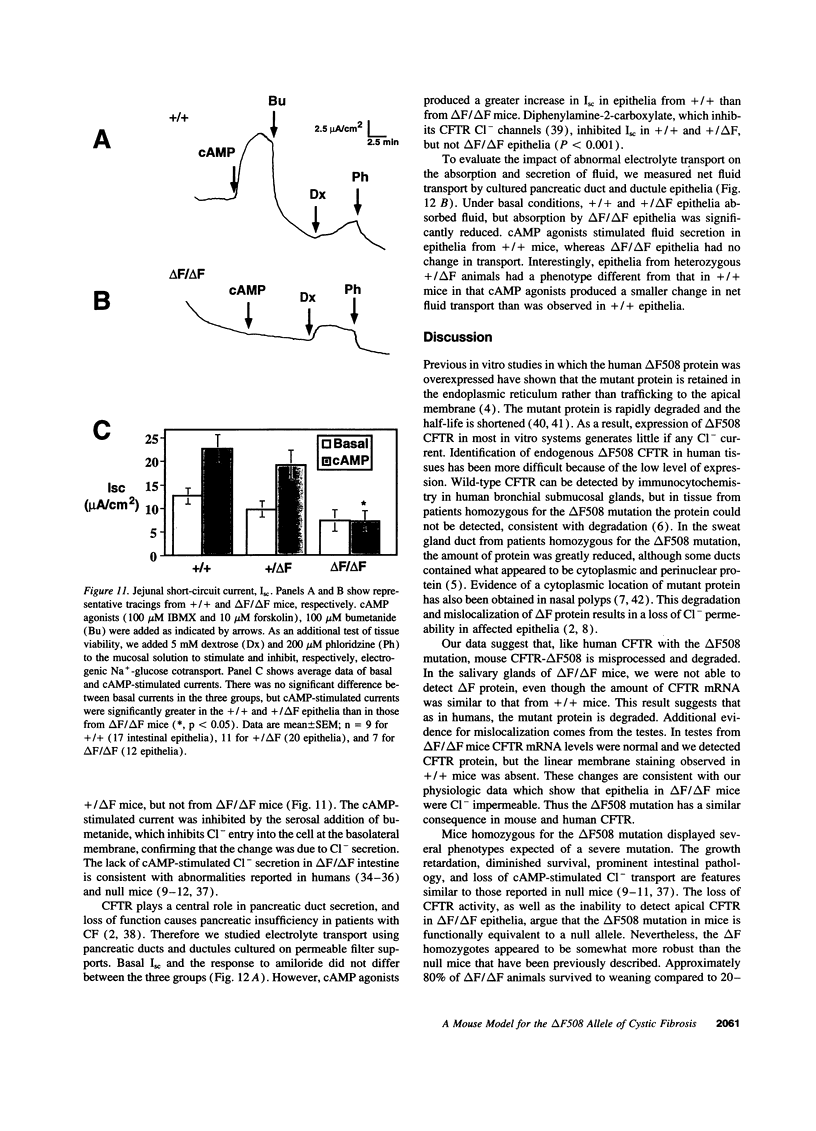
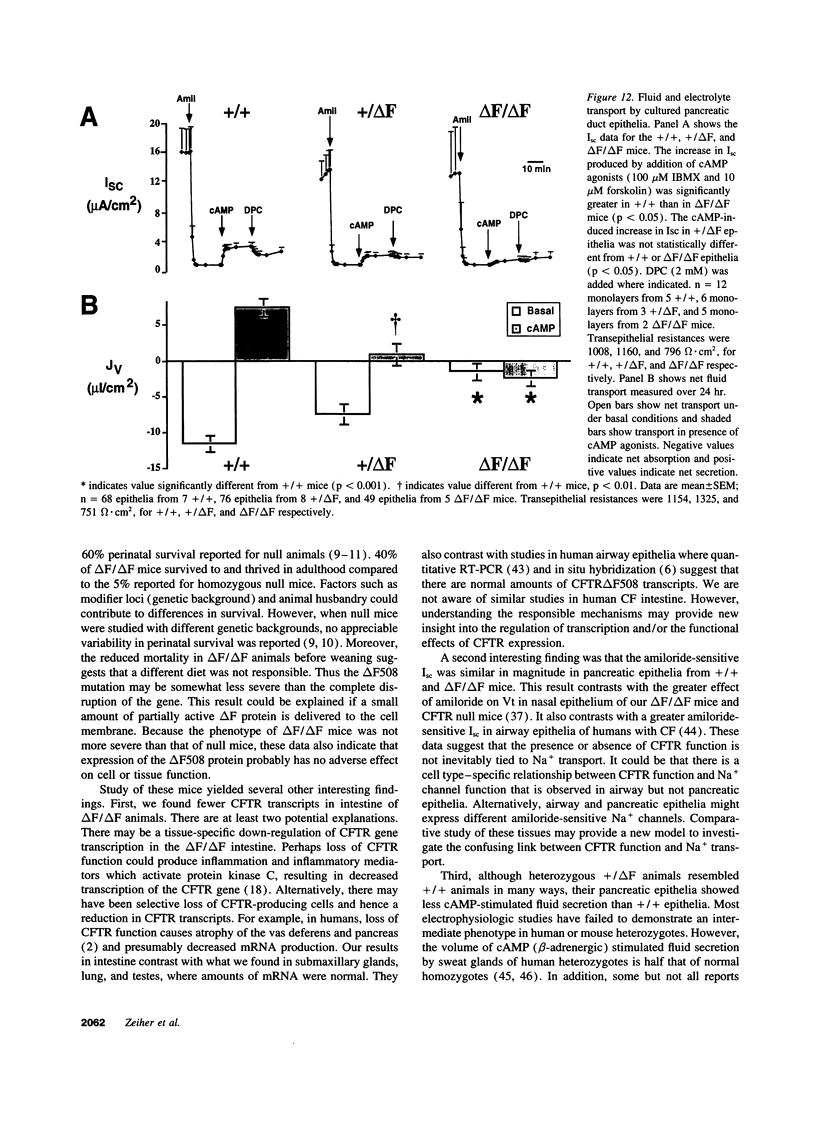
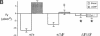Abstract
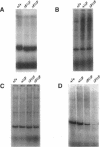
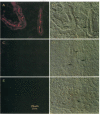
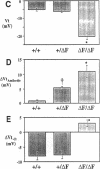
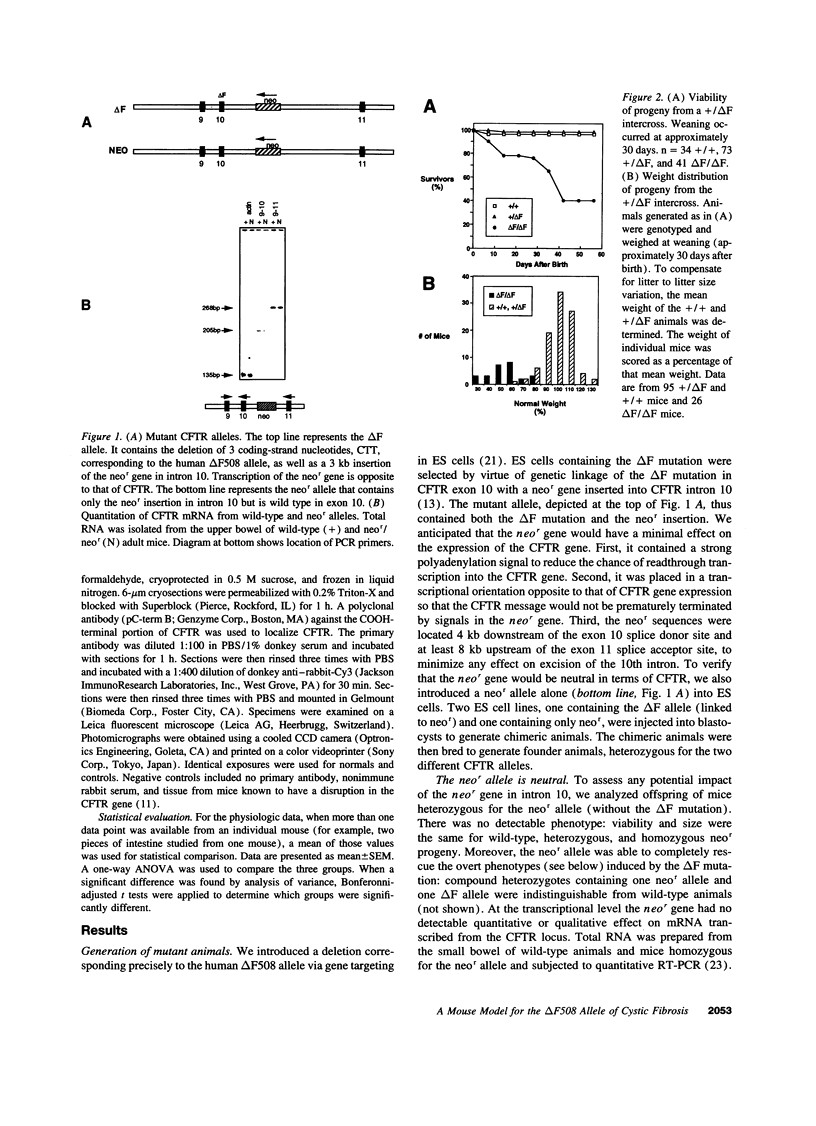
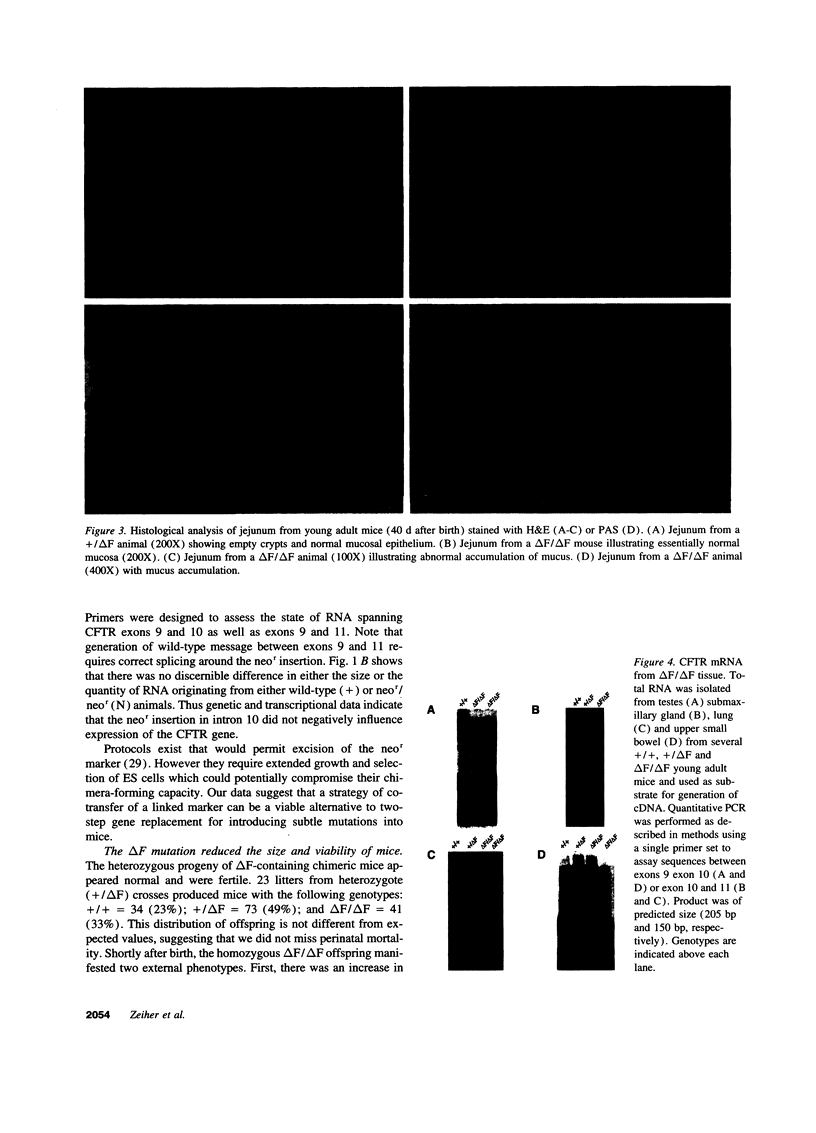
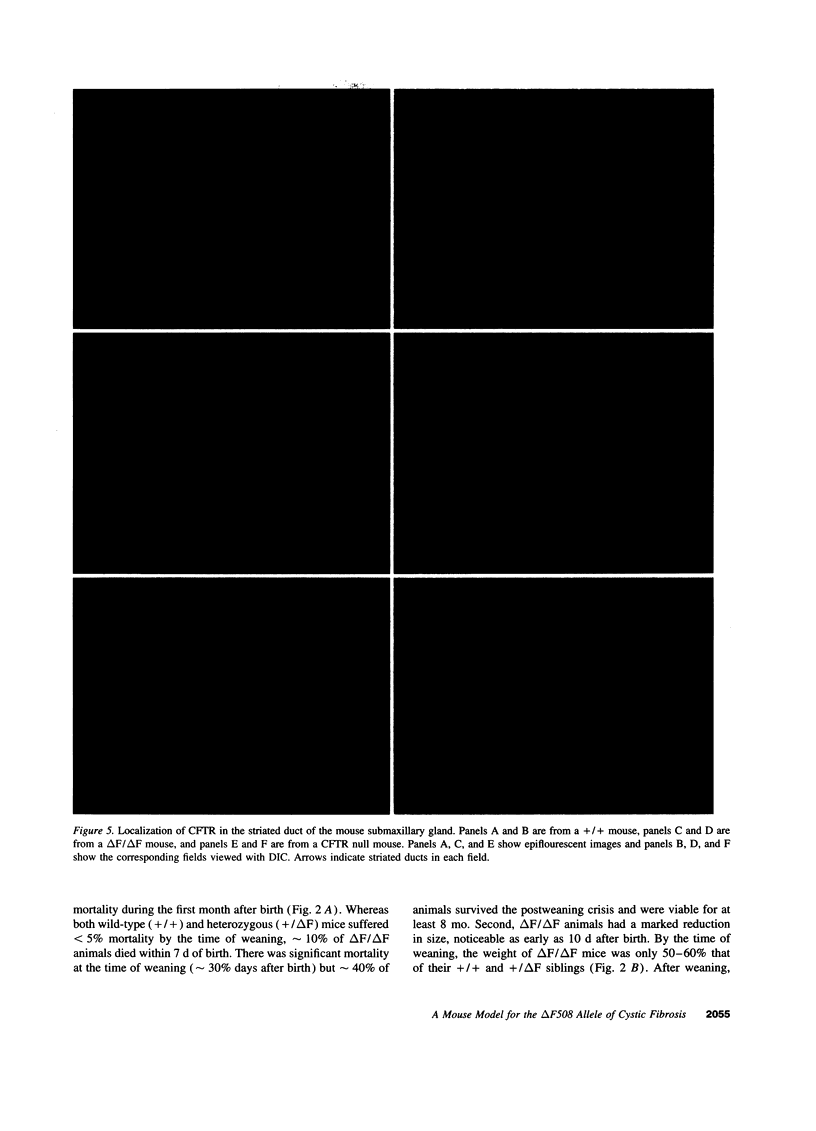
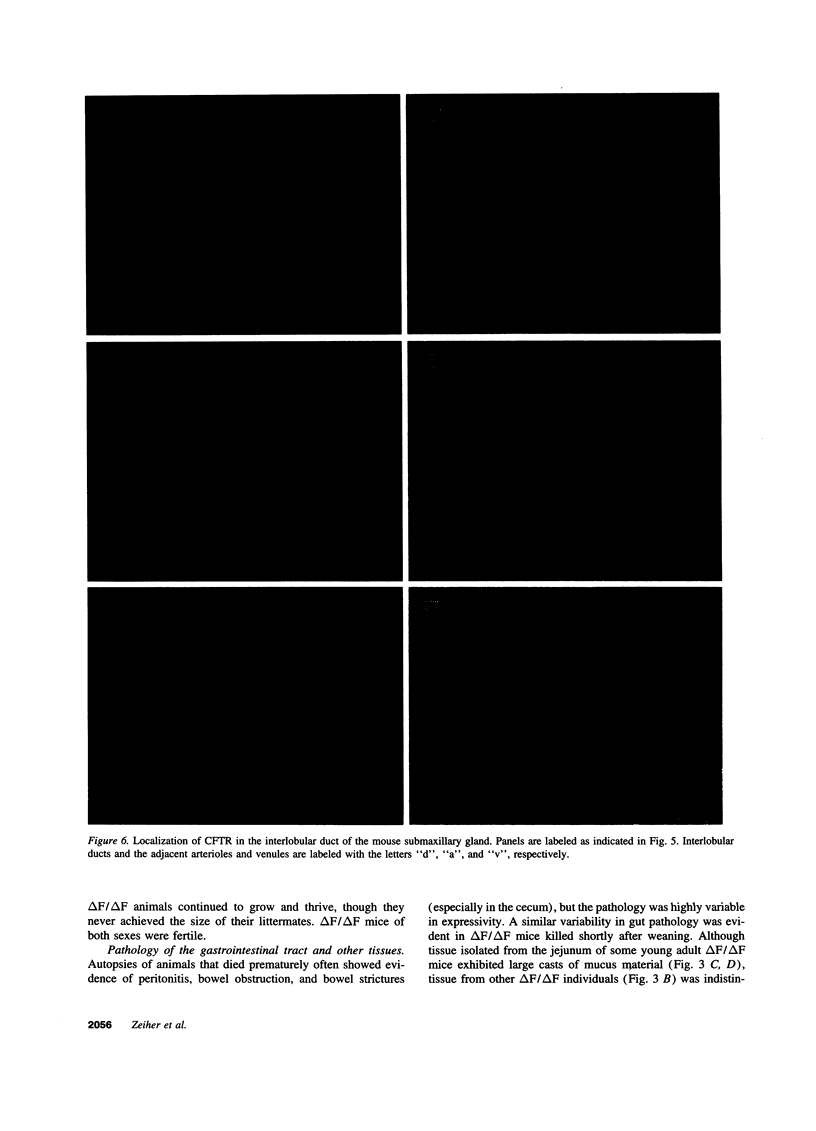
The most common cause of cystic fibrosis is a mutation that deletes phenylalanine 508 in cystic fibrosis transmembrane conductance regulator (CFTR). The delta F508 protein is misprocessed and degraded rather than traveling to the apical membrane. We used a novel strategy to introduce the delta F508 mutation into the mouse CFTR gene. Affected epithelia from homozygous delta F508 mice lacked CFTR in the apical membrane and were Cl-impermeable. These abnormalities are the same as those observed in patients with delta F508 and suggest that these mice have the same cellular defect. 40% of homozygous delta F508 animals survived into adulthood and displayed several abnormalities found in human disease and in CFTR null mice. These animals should provide an excellent model to investigate pathogenesis and to examine therapies directed at correcting the delta F508 defect.
Full text
PDF

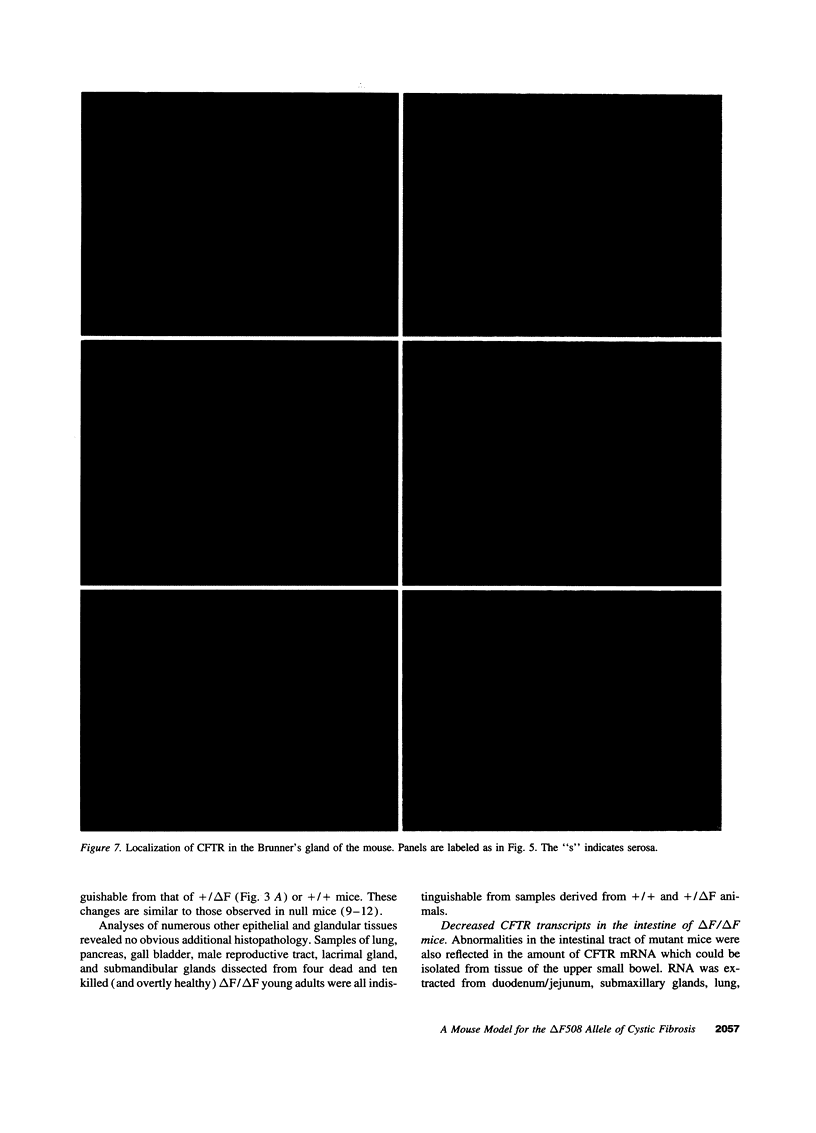
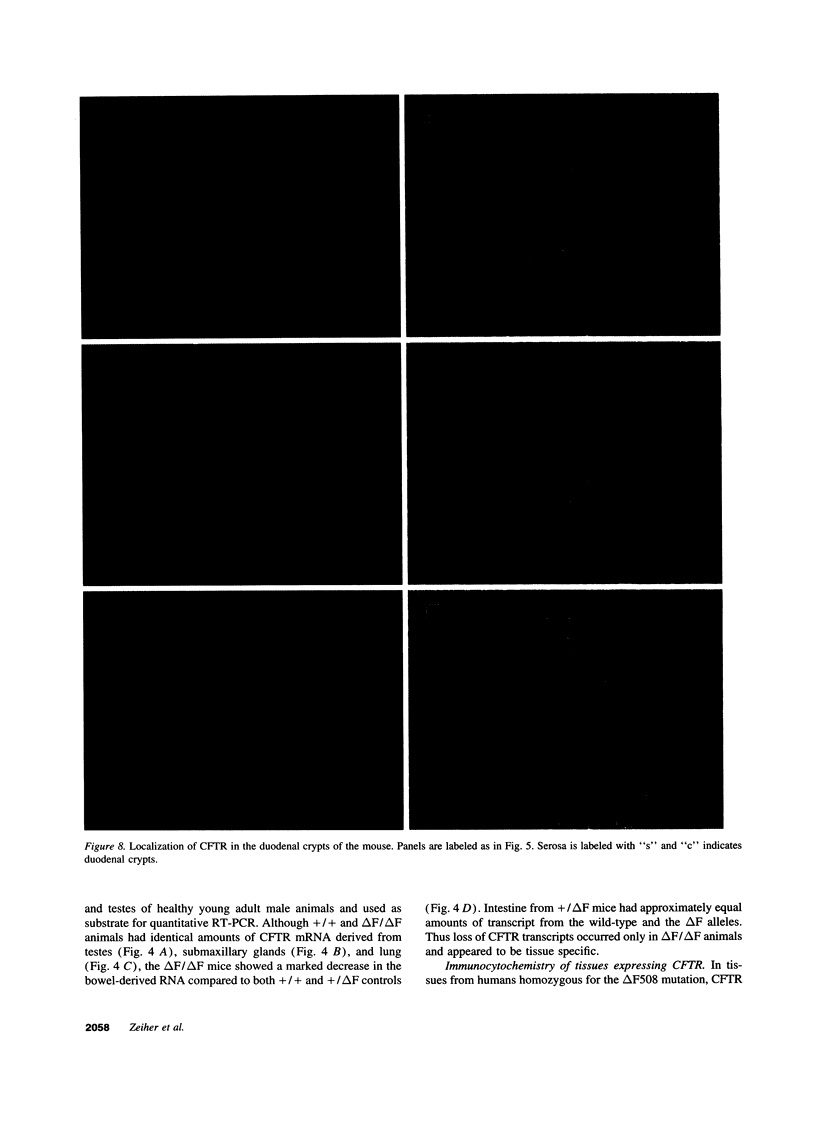
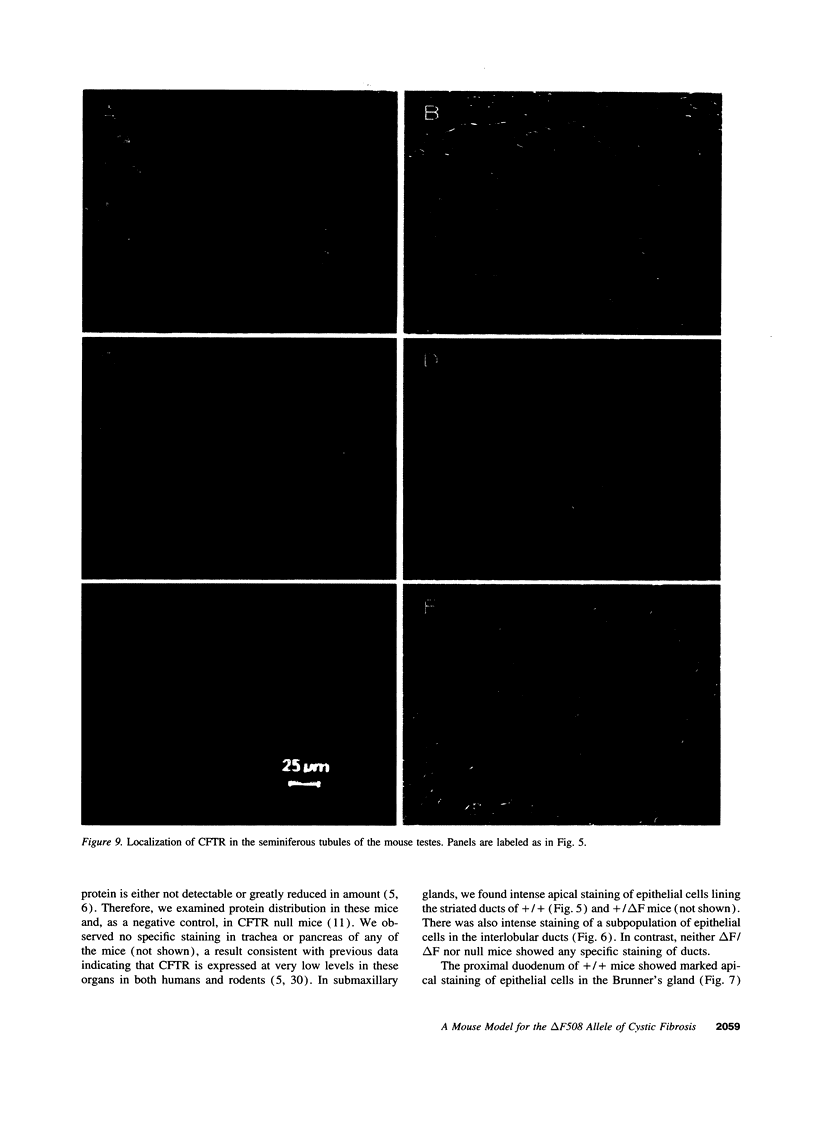
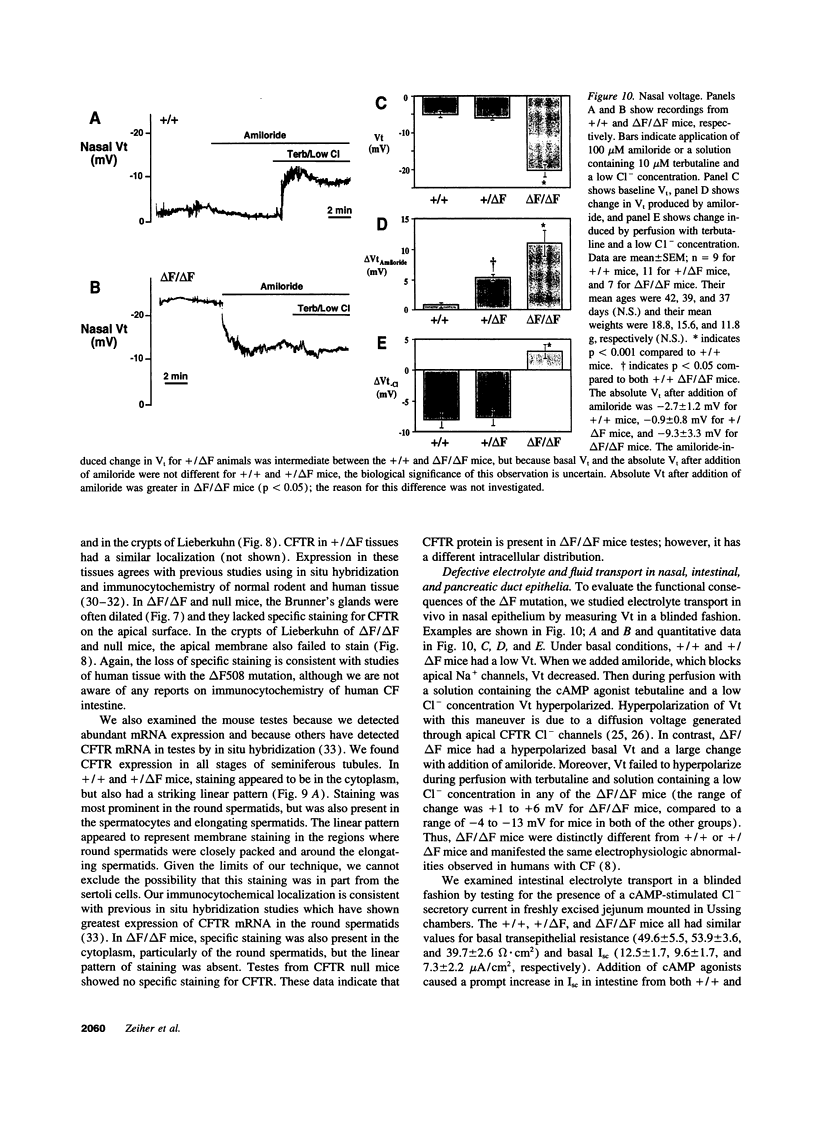


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton E. W., Currie D., Logan-Sinclair R., Warner J. O., Hodson M. E., Geddes D. M. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J. 1990 Sep;3(8):922–926. [PubMed] [Google Scholar]
- Behm J. K., Hagiwara G., Lewiston N. J., Quinton P. M., Wine J. J. Hyposecretion of beta-adrenergically induced sweating in cystic fibrosis heterozygotes. Pediatr Res. 1987 Sep;22(3):271–276. doi: 10.1203/00006450-198709000-00007. [DOI] [PubMed] [Google Scholar]
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Clarke L. L., Grubb B. R., Gabriel S. E., Smithies O., Koller B. H., Boucher R. C. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 1992 Aug 21;257(5073):1125–1128. doi: 10.1126/science.257.5073.1125. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Halstead J., Ratcliff R., Colledge W. H., Evans M. J. The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J Physiol. 1995 Jan 15;482(Pt 2):449–454. doi: 10.1113/jphysiol.1995.sp020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Deng C., Thomas K. R., Capecchi M. R. Location of crossovers during gene targeting with insertion and replacement vectors. Mol Cell Biol. 1993 Apr;13(4):2134–2140. doi: 10.1128/mcb.13.4.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Ostedgaard L. S., Welsh M. J. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol. 1992 Aug;118(3):551–559. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorin J. R., Dickinson P., Alton E. W., Smith S. N., Geddes D. M., Stevenson B. J., Kimber W. L., Fleming S., Clarke A. R., Hooper M. L. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992 Sep 17;359(6392):211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yankaskas J. R., Ernst S. A., Yang Y., Marino C. R., Boucher R. C., Cohn J. A., Wilson J. M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992 Nov;2(3):240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Gabriel S. E., Brigman K. N., Koller B. H., Boucher R. C., Stutts M. J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994 Oct 7;266(5182):107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- Gaillard D., Ruocco S., Lallemand A., Dalemans W., Hinnrasky J., Puchelle E. Immunohistochemical localization of cystic fibrosis transmembrane conductance regulator in human fetal airway and digestive mucosa. Pediatr Res. 1994 Aug;36(2):137–143. doi: 10.1203/00006450-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Githens S., Schexnayder J. A., Moses R. L., Denning G. M., Smith J. J., Frazier M. L. Mouse pancreatic acinar/ductular tissue gives rise to epithelial cultures that are morphologically, biochemically, and functionally indistinguishable from interlobular duct cell cultures. In Vitro Cell Dev Biol Anim. 1994 Sep;30A(9):622–635. doi: 10.1007/BF02631262. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Shapiro A. B., Rao M. C., Layden T. J. In vivo evidence of altered chloride but not potassium secretion in cystic fibrosis rectal mucosa. Gastroenterology. 1991 Oct;101(4):1012–1019. doi: 10.1016/0016-5085(91)90728-4. [DOI] [PubMed] [Google Scholar]
- Kartner N., Augustinas O., Jensen T. J., Naismith A. L., Riordan J. R. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat Genet. 1992 Aug;1(5):321–327. doi: 10.1038/ng0892-321. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Clarke L. L., Boucher R. C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991 Aug 22;325(8):533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemna W. K., Feldman G. L., Kerem B., Fernbach S. D., Zevkovich E. P., O'Brien W. E., Riordan J. R., Collins F. S., Tsui L. C., Beaudet A. L. Mutation analysis for heterozygote detection and the prenatal diagnosis of cystic fibrosis. N Engl J Med. 1990 Feb 1;322(5):291–296. doi: 10.1056/NEJM199002013220503. [DOI] [PubMed] [Google Scholar]
- Lukacs G. L., Chang X. B., Bear C., Kartner N., Mohamed A., Riordan J. R., Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993 Oct 15;268(29):21592–21598. [PubMed] [Google Scholar]
- Mansour S. L., Goddard J. M., Capecchi M. R. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993 Jan;117(1):13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- Marino C. R., Matovcik L. M., Gorelick F. S., Cohn J. A. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991 Aug;88(2):712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty N. A., McDonough S., Cohen B. N., Riordan J. R., Davidson N., Lester H. A. Voltage-dependent block of the cystic fibrosis transmembrane conductance regulator Cl- channel by two closely related arylaminobenzoates. J Gen Physiol. 1993 Jul;102(1):1–23. doi: 10.1085/jgp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal W. K., Hasty P., McCray P. B., Jr, Casey B., Rivera-Pérez J., Welsh M. J., Beaudet A. L., Bradley A. A severe phenotype in mice with a duplication of exon 3 in the cystic fibrosis locus. Hum Mol Genet. 1993 Oct;2(10):1561–1569. doi: 10.1093/hmg/2.10.1561. [DOI] [PubMed] [Google Scholar]
- Puchelle E., Gaillard D., Ploton D., Hinnrasky J., Fuchey C., Boutterin M. C., Jacquot J., Dreyer D., Pavirani A., Dalemans W. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am J Respir Cell Mol Biol. 1992 Nov;7(5):485–491. doi: 10.1165/ajrcmb/7.5.485. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Zheng H., Whiting J., Krumlauf R., Bradley A. Hoxb-4 (Hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993 Apr 23;73(2):279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Cuthbert A. W., MacVinish L. J., Foster D., Anderson J. R., Colledge W. H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat Genet. 1993 May;4(1):35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Variable reduction in beta-adrenergic sweat secretion in cystic fibrosis heterozygotes. J Lab Clin Med. 1988 May;111(5):511–518. [PubMed] [Google Scholar]
- Smith J. J., Welsh M. J. Fluid and electrolyte transport by cultured human airway epithelia. J Clin Invest. 1993 Apr;91(4):1590–1597. doi: 10.1172/JCI116365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snouwaert J. N., Brigman K. K., Latour A. M., Malouf N. N., Boucher R. C., Smithies O., Koller B. H. An animal model for cystic fibrosis made by gene targeting. Science. 1992 Aug 21;257(5073):1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Strong T. V., Boehm K., Collins F. S. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994 Jan;93(1):347–354. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. S., Weis J. H. Development of a sensitive reverse transcriptase PCR assay, RT-RPCR, utilizing rapid cycle times. PCR Methods Appl. 1992 Nov;2(2):137–143. doi: 10.1101/gr.2.2.137. [DOI] [PubMed] [Google Scholar]
- Tata F., Stanier P., Wicking C., Halford S., Kruyer H., Lench N. J., Scambler P. J., Hansen C., Braman J. C., Williamson R. Cloning the mouse homolog of the human cystic fibrosis transmembrane conductance regulator gene. Genomics. 1991 Jun;10(2):301–307. doi: 10.1016/0888-7543(91)90312-3. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Chu C. S., Paakko P. K., Banks T. C., Yoshimura K., Ferrans V. J., Chernick M. S., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise A. E., Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991 Oct 3;353(6343):434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Trezise A. E., Linder C. C., Grieger D., Thompson E. W., Meunier H., Griswold M. D., Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat Genet. 1993 Feb;3(2):157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- Veeze H. J., Sinaasappel M., Bijman J., Bouquet J., de Jonge H. R. Ion transport abnormalities in rectal suction biopsies from children with cystic fibrosis. Gastroenterology. 1991 Aug;101(2):398–403. doi: 10.1016/0016-5085(91)90017-f. [DOI] [PubMed] [Google Scholar]
- Ward C. L., Kopito R. R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994 Oct 14;269(41):25710–25718. [PubMed] [Google Scholar]
- Yorifuji T., Lemna W. K., Ballard C. F., Rosenbloom C. L., Rozmahel R., Plavsic N., Tsui L. C., Beaudet A. L. Molecular cloning and sequence analysis of the murine cDNA for the cystic fibrosis transmembrane conductance regulator. Genomics. 1991 Jul;10(3):547–550. doi: 10.1016/0888-7543(91)90434-g. [DOI] [PubMed] [Google Scholar]