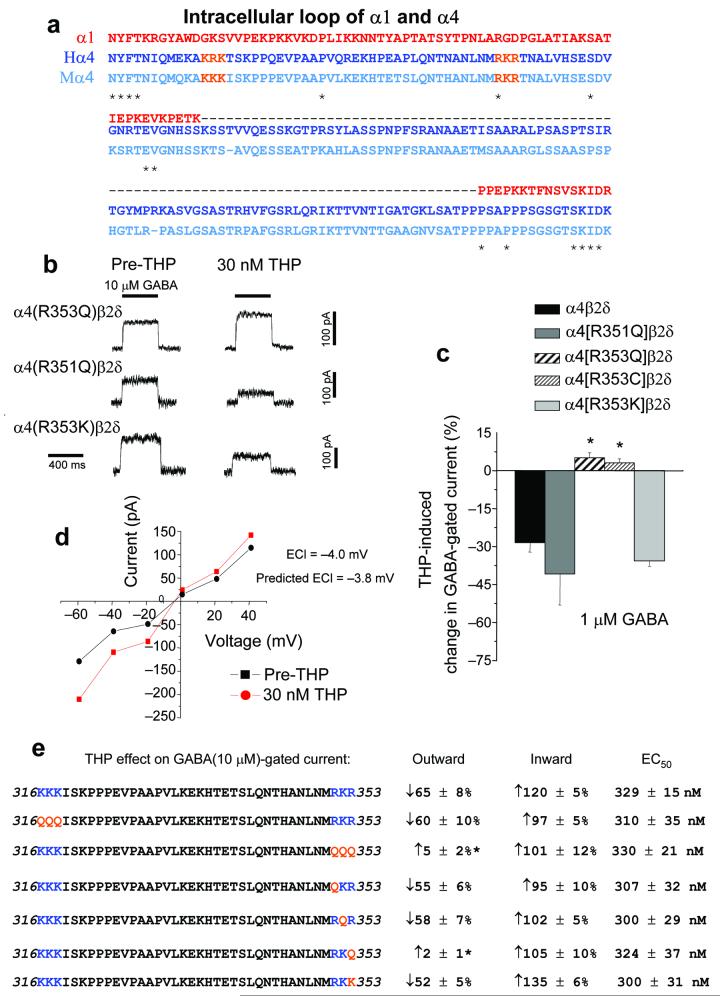
Figure 2.
Arginine 353 in the α4 subunit is necessary for the direction-sensitive inhibition of α4β2δ GABAA receptors by THP. (a) Alignment of the intracellular loop of α1 and α4 (H, human; M, mouse) subunits reveals limited identity (< 10%). *identical residues for all three. (The sequences for human and mouse α1 are identical.) Orange, residues to be mutated. (b) Representative traces showing the effect of 30 nM THP on GABA(10 μM)-gated current at the indicated mutated α4β2δ GABAA receptors. Basic arginine (R351 or R353) residues in the α4 subunit were mutated to a neutral glutamine (Q) and/or a basic lysine (K). (c) Averaged data, Effects of 30 nM THP at α4β2δ receptors containing wild-type or mutant α4 subunits on outward GABA(1 μM)-gated current. (n = 4 – 5 cells for each group, *P < 0.05 versus wild-type α4β2δ). (d) Current-voltage plot recorded from α4[R353Q]β2δ GABAA receptors before and after THP; ECl = −4.0 mV; predicted ECl = −3.8 mV, averaged from 5 cells for each point. (e) Summary diagram. Left, amino acid sequences 316-353 within the mouse α4 loop (basic residues, blue; mutated residues, orange). The two regions of the α4 loop with consecutive basic residues (316 – 318 and 351 – 353, in blue) were mutated as a group or singly to a neutral glutamine (Q in red) or to a basic lysine (K). Right, Effects of the indicated mutation on outward and inward GABA(10 μM)-gated current are indicated, as is the GABA EC50 (Mean ± SEM). All mutations produced current of similar magnitude (100 – 200 pA; n = 5 – 6 cells for each group).