Figure 3.
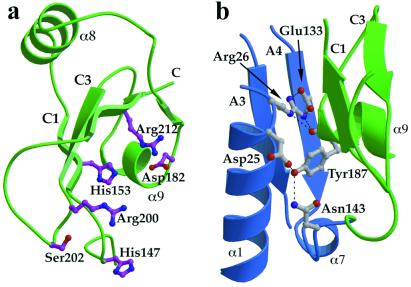
(a) The C-terminal domain contains a number of residues that are highly conserved from archaea to humans. A cluster of invariant polar amino acids (mostly positively charged) are located on one surface of the domain. (b) The subunit interface contains few hydrophobic residues and is instead stabilized by polar interactions between a number of invariant residues in the N-terminal domain and Tyr 187 in the C-terminal domain. The figure shows the conserved residues at the interface and the hydrogen-bonding interactions. The secondary structure elements are colored as in Fig. 2.