Abstract
The actions of the protonophore CCCP on intracellular Ca2+ regulation and exocytosis in chromaffin cells have been examined. Simultaneous fura-2 imaging and amperometry reveal that exposure to CCCP not only perturbs mitochondrial function but that it also alters vesicular storage of Ca2+ and catecholamines. By disrupting the pH gradient of the secretory vesicle membrane, the protonophore allows both Ca2+ and catecholamine to leak into the cytosol. Unlike the high cytosolic Ca2+ concentrations resulting from mitochondrial membrane disruption, Ca2+ leakage from secretory vesicles may initiate exocytotic release. In conjunction with previous studies, this work reveals that catalytic and self-sustained vesicular Ca2+-induced exocytosis occurs with extended exposure to weak acid or base protonophores.
Introduction
Elevated intracellular Ca2+ triggers exocytosis from secretory vesicles. The primary route for its elevation is entry of external Ca2+ via voltage-gated Ca2+ channels[1]. However, intracellular stores of Ca2+ can also promote exocytosis [2–5]. The endoplasmic reticulum is often designated as the key dynamic Ca2+ reservoir within cells, participating in Ca2+-induced Ca2+ release and using Ca2+-regulated enzymes[6–8]. Another source of intracellular Ca2+ is the secretory vesicles where Ca2+ is sequestered into the acidic compartment and stored at high concentration by virtue of an association with chromogranin A[9, 10]. In fact, nearly 60% of Ca2+ in chromaffin cells resides in the vesicular pools[11] although >99% of the vesicular Ca2+ is highly associated within the other vesicular contents[12]. Weak bases such as methylamine and methamphetamine, as well as VMAT inhibitors, disrupt vesicular storage, promoting Ca2+ leakage from the vesicles and evoking exocytosis[13, 14]. Ca2+ release from vesicles in many cell types such as insulin-secreting cells, buccal ganglion neurons, caudate nucleus synaptosomes, and neurohypophysial nerve terminals has been demonstrated[3–5]. While high affinity Ca2+ probes localized within vesicles of PC-12 cells revealed that the vesicular compartment tracks cytosolic Ca2+ levels,[15] other vesicular compartments are known to have Ca2+ concentrations that are highly sensitive to the vesicular pH. This vesicular Ca2+ source is particularly intriguing because it suggests that vesicles can directly induce their own release[16].
A third intracellular store of Ca2+ is mitochondria. Mitochondria accumulate Ca2+ due to a pH gradient between the inner mitochondrial membrane (pH=6.8) and the mitochondrial matrix (pH = 8)[17]. Several investigators have shown that mitochondria are the chief organelle that restore elevated levels of intracellular Ca2+ to the resting concentration[18–21]. A common strategy to probe mitochondrial function is to use uncouplers, substances that dissipate the pH gradient, such as carbonyl cyanide m-chlorophenylhydrazone (CCCP). In this work, we examine the actions of the protonophore CCCP on intracellular Ca2+ regulation and exocytosis in chromaffin cells. CCCP has one extractable proton (pKa ~ 6[22]) and is able to cross lipophilic membranes in both the protonated and deprotonated forms. CCCP acts as a proton shuttle by bringing protons from the cytosol to the more basic mitochondrial matrix, thus collapsing the pH gradient that acts as the driving force for mitochondrial Ca2+ uptake. While CCCP can be used to investigate the role of mitochondrial Ca2+ regulation, recent evidence indicates that structurally similar compounds can also cause Ca2+ leakage from vesicles in PC12 cells[12]. Since vesicular Ca2+ leakage is often accompanied by dissipation of the proton gradient and self-catalyzed exocytosis[13,14], we felt it was imperative to evaluate whether CCCP could evoke exocytosis as well.
Methods
Acutely dissociated bovine adrenal medullary chromaffin cells were prepared as previously described[23]. The cells were loaded with the cytosolic Ca2+-chelating dye, fura-2 before simultaneously recording fluorescence and release with amperometry at carbon-fiber microelectrodes[23]. The cells were exposed to 2 μM CCCP either by transient pressure ejection for 3 seconds or incubation for 5 or 15 minutes. Except where noted, cells were maintained in a standard buffer solution containing 10 mM TRIS·HCl, 150 mM NaCl, 5 mM KCl, 2 mM CaCl2·2H2O, 1.2 mM MgCl2·6H2O, and 5 mM glucose, adjusted to pH 7.4. Carbon-fiber disk microelectrodes were beveled at 45º. Amperometry employed an Axopatch 200A potentiostat with an applied voltage of +650 V versus a Ag/AgCl reference electrode. Release was stimulated with 3 s applications of 60 mM K+. Cytosolic catecholamine measurements were performed in Ca2+-free buffer using transient applications of 10 μM digitonin[14].
Results and Discussion
Transient CCCP Exposure Can Evoke Cytosolic Ca2+ and Exocytosis
To examine the direct, short-term effects of the protonophore, CCCP was pressure ejected onto individual chromaffin cells in Ca2+-free buffer. As shown for a single example in Figure 1, a 3 second application of CCCP evoked elevated cytosolic Ca2+, measured via fura-2 fluorescence, in all cases. One source of this Ca2+ is undoubtedly intracellular mitochondria where CCCP uncouples the proton gradient that controls mitochondrial stores of Ca2+. Recent results have shown that compounds similar to CCCP can also displace intravesicular Ca2+, a process that appears to be controlled by a H+/Ca2+ exchanger[12]. Thus, raising the vesicular pH via CCCP would generate conditions where the chromogranin A matrix could begin to unravel, allowing vesicular Ca2+ to escape into the cytosol via the exchanger. The elevated cytosolic Ca2+ concentration was sufficient to cause vesicular release, measured by amperometry, in two out of ten cells tested in this way (bottom amperometric trace in Figure 1).
Figure 1. Amperometric and fura-2 signal traces in response to a transient application of CCCP.
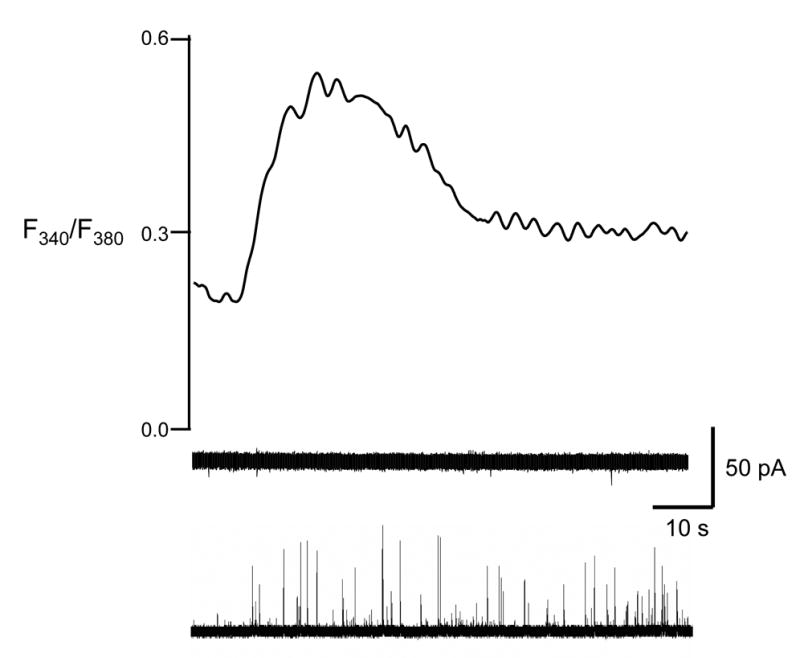
A 3-s application of 2 μM CCCP caused Ca2+ elevation (top). In 8 out of 10 cells, no release was observed (middle). In 2 out of 10 cells, the elevated Ca2+ concentration was sufficient to evoke amperometric release (bottom). The right y-axis corresponds to the upper trace while the scale on the left corresponds to both amperometric traces. Cells were maintained in Ca2+-free buffer.
Long-Term CCCP Exposure Increases Cytosolic Ca2+ Clearance and Release Duration
When examined following a 5 minute incubation in CCCP (2 μM) in buffer containing Ca2+, levels of intracellular Ca2+ were returned to levels similar to control cells. However, spontaneous exocytotic events were occasionally observed during the CCCP incubation period (data not shown). These spontaneous events occurred at a lower frequency than stimulated events and were more common at the beginning of the incubation period than the end. More dramatic effects were seen on Ca2+ clearance following membrane depolarization. Incubation in CCCP increased both the cytosolic Ca2+ clearance time and the catecholamine release duration evoked by transient K+, consistent with previous results[18, 21]. Specifically, responses to pressure ejection (3 s) of 60 mM K+ at cells incubated for 5 minutes in CCCP (2 μM, n=6) were compared to control cells (n=6) stimulated in the same way (Figure 2). The cytosolic Ca2+ clearance in the cells exposed to CCCP had a half-width of 20.3 ± 2.3 s. This was almost twice as long as the Ca2+ clearance in the control cells, with a fura-2 half-width of 9.4 ± 1.2 s (Figure 2). Similarly, the 21.9 ± 1.9 s duration of vesicular release from cells maintained in buffer containing CCCP was more than twice the 5.6 ± 0.7 s duration observed in control cells. The increase in both the calcium clearance time and catecholamine release duration was significantly different from the control values (Mann-Whitney U-test, p < 0.01). Thus, during K+ evoked release, we largely attribute the effect of CCCP on elevated Ca2+ and release duration to its effects on mitochondria, where its uncoupling impairs the ability to restore cytosolic Ca2+ levels. This conclusion is consistent with that of Yang and coworkers who reported that the Na+/Ca2+ exchanger, endoplasmic reticulum, and mitochondria work cooperatively to regulate Ca2+ near exocytotic sites but that the mitochondrial uptake is dominant when the cytosolic Ca2+ diffuses away from exocytotic sites[18].
Figure 2. Amperometric and fura-2 signal traces in the presence and absence of the protonophore, CCCP.
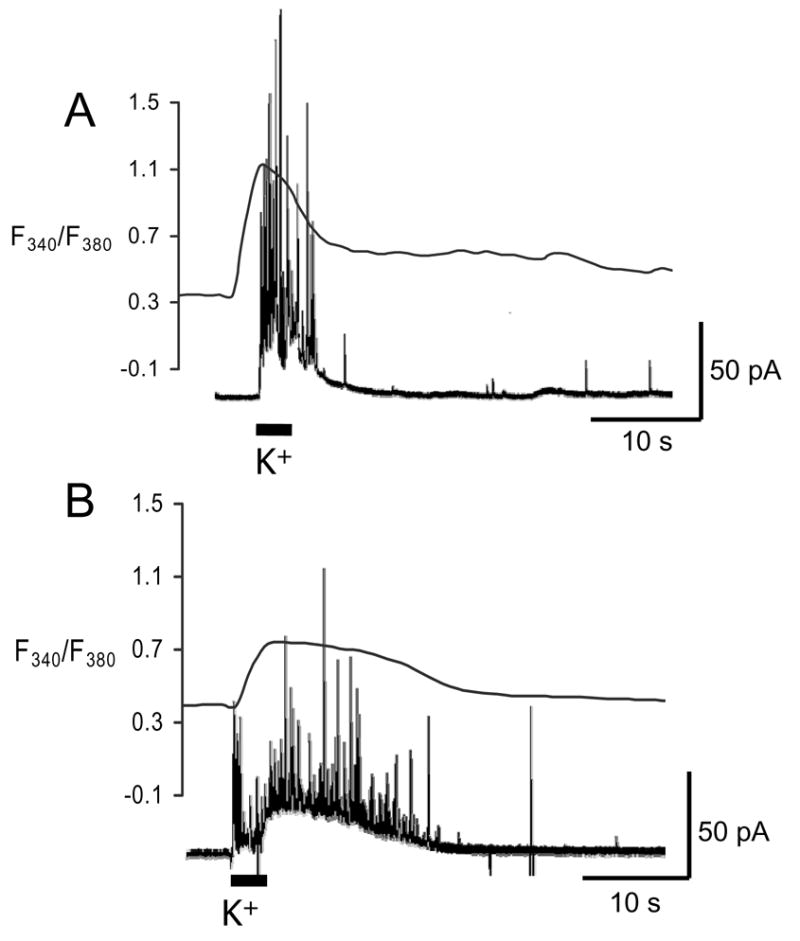
Each cell was exposed to a 3-s pressure ejection of 60 mM K+ in Ca2+ containing buffer. Overlays of representative amperometric (left y-axis) and fura-2 (right y-axis) traces for A) untreated cells and B) treated cells. Exposure to CCCP increased both the release duration and the width at half-maximal height of the fura-2 signal.
Quantal Size Decreases with Long Protonophore Exposure Time
The role of CCCP in secretory vesicle leakage was examined by monitoring the quantal size (Q) of exocytosed catecholamines with different CCCP exposure times. Each cell was stimulated 4 times by pressure ejection of high K+ with 2 minute intervals between each stimulation, and the resulting exocytotic events were recorded. Next, the cell was incubated in CCCP (2 μM) for 5 or 15 minutes. After washing with CCCP-free buffer, the same stimulation pattern with high K+ was applied again. To account for the variability that can exist between cells, each cell was used as its own control by normalizing amperometric spikes obtained post-incubation with the spikes obtained prior to the CCCP incubation. In control cells (n=10), neither 5 nor 15 minute incubations in CCCP-free buffer caused a significant change in spike half-width (t1/2) or Q as indicated by post/pre ratios near 1.0 for all cases (Figure 3B and 3C, black bars). Ratios calculated from amperometric traces recorded before and after a 5 min incubation in CCCP (n=4) also did not yield significantly different t1/2 compared to control cells (Figure 3B, white bars). The Q ratios were slightly lower, but the result was not significant (p > 0.05). In contrast, Q ratios calculated for cells that were incubated with CCCP for 15 minutes (n=5) are significantly smaller (0.51 post/pre incubation) than control cell Q ratios (Figure 3C, white bars) while t1/2 ratios remain unaffected. Because there was not a significant difference between the t1/2 ratios obtained following incubation with CCCP, it is clear that the lowered amounts released per exocytotic event are not due to an increased affinity between catecholamine and chromogranin A. Rather, the results are consistent with a disruption of vesicular storage following incubation with CCCP as a consequence of collapsing intracellular pH gradients, allowing catecholamine molecules and Ca2+ leakage. This would result in the reduced amount released in each exocytotic event, as observed.
Figure 3. Amperometric detection of time-dependent depletion of epinephrine from vesicles.
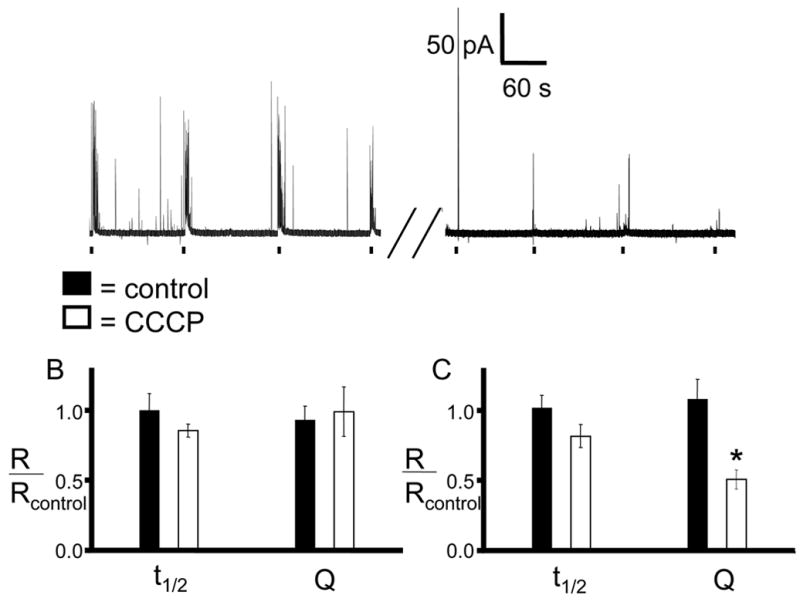
Individual chromaffin cells were exposed 4 times to a 3-s pressure ejection of 60 mM K+in a Ca2+ containing buffer. The cells were then incubated in buffer containing 2 μM CCCP for the indicated time. After the incubation period, the cells were washed with buffer and stimulated again using the same K+ ejection pattern. Average spike parameters were then normalized by the pre-incubation values for each cell so that each cell acted as its own control. A) A representative amperometric trace of a 15 minute CCCP incubation. B) 5 minute incubation in CCCP caused no significant change in the average current spike half-width or quantal size compared with pre-incubation averages. C) 15 minute incubation causes a significant decrease in quantal size (p < 0.001 by Mann-Whitney U-test).
Cytosolic Catecholamine Increases Dramatically with Long CCCP Exposure Time
Because both catecholamine and Ca2+ are highly associated within the acidic intravesicular matrix, leakage of catecholamine into the cytosol during CCCP incubation is consistent with the simultaneous release of intravesicular Ca2+ reported previously[12]. This leakage was verified by examining cytosolic catecholamine molecules after permeabilizing CCCP-exposed cells with 10 μM digitonin in Ca2+-free buffer. Unlike the millisecond timescale of exocytotic release, cytosolic catecholamine liberated following digitonin permeation has a time course of several seconds (Figure 4). In control cells where CCCP was not included in the buffer, no catecholamine was detected when the cells were exposed to digitonin (data not shown), indicating low cytoplasmic catecholamine levels. However, digitonin permeabilization after 5-minute CCCP (2 μM) incubation resulted in detection of 2.1 ± 0.5 x 107 cytoplasmic catecholamine molecules (n=7) and 5.5 ± 1.0 x 107 cytoplasmic catecholamine molecules were detected from cells incubated for 15 minutes with the same concentration of CCCP (n=6). These cytosolic catecholamine amounts are comparable to those measured when chromaffin cells were incubated with the VMAT inhibitor, reserpine[14]. Thus, the cytosolic catecholamine measurements clearly illustrate that the intravesicular contents can be displaced into the cytosol following CCCP. This extensive vesicular perturbation after 15 minute CCCP incubation is manifested by the decreased amounts released in each exocytotic event (Figure 3C).
Figure 4. Cytosolic catecholamine content evaluated by digitonin permeabilization.
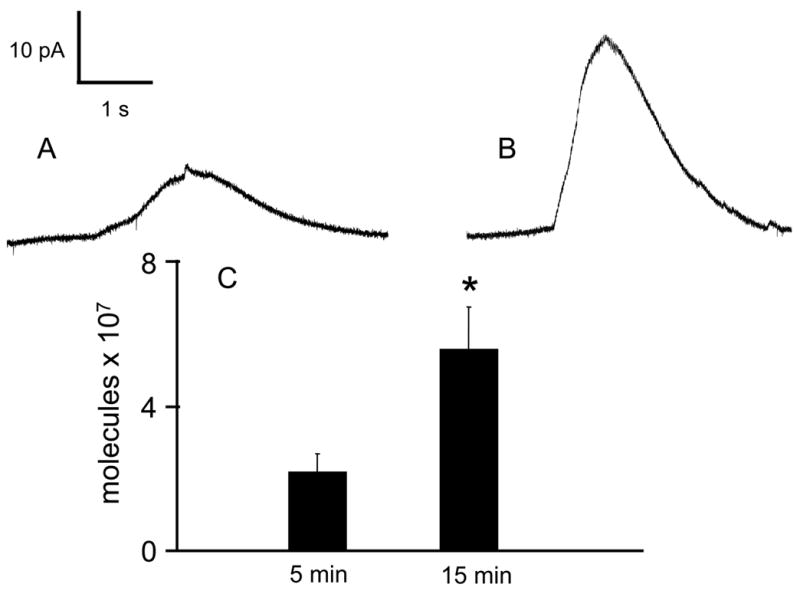
The cells were incubated with 2 μM CCCP in Ca2+-containing buffer for the either A) 5 minutes or B) 15 minutes. After washing and transferring to Ca2+-free buffer, 10 μM digitonin was pressure ejected onto the cell. The resulting envelope is an indication of the amount of catecholamine that was present in the cytosol of the cell. C) The 15 minute incubation resulted in approximately double the amount of catecholamine molecules that were detected after a 5 minute incubation (p < 0.05 by Mann-Whitney U-test).
Conclusions
The protonophore CCCP has been a critical tool in establishing the predominance of mitochondria in restoring high cytosolic Ca2+ to resting levels[20, 21, 24]. Simultaneous fura-2 imaging and amperometry used in this work reveal that incubations in CCCP also alter vesicular storage of Ca2+ and catecholamine. These data clearly indicate that CCCP promotes leakage of vesicle contents into the cytosol. Indeed, the magnitude of the change in intracellular Ca2+ concentration following transient exposure to CCCP is similar to when the cells were depolarized (compare Figures 1 and 2). Despite evoking large cytosolic Ca2+ concentrations, exposure to CCCP did not always cause secretion, and it did so primarily upon the initial exposure. Elevated intracellular Ca2+ can only cause exocytosis if it occurs in close proximity to both the cell membrane and a fusion competent vesicle. Because CCCP disrupts pH gradients, and thus Ca2+ storage, at multiple intracellular organelles, most of which are not proximal to the cell membrane, overall intracellular Ca2+ rises but only a small portion of it is optimally positioned to induce exocytosis. Based on this work, previous results achieved with amine weak bases[14] can be further generalized to state that the weak acids as well as weak base protonophores can induce unraveling of vesicular contents. The results also show that the vesicular Ca2+ likely catalyzes and promotes self-sustained exocytosis.
Acknowledgments
This work was supported by National Institutes of Health grant NS 38879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsien RW. Calcium Channels in Excitable Cell Membranes. Ann Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- 2.Yoo SH, Albanesi JP. Ca2+-Induced Conformational Change and Aggregation of Chromogranin A. J Bio Chem. 1990;265:14414–14421. [PubMed] [Google Scholar]
- 3.Troadec JD, Thirion S, Laugier JP, Nicaise G. Calcium-Induced Calcium Increase in Secretory Vesicles of Permeabilized Rat Neurohypophysial Nerve. Terminals Bio Cell. 1998;90:339–349. [PubMed] [Google Scholar]
- 4.Scheenen WJJM, Wollheim CB, Pozzan T, Fasolato C. Ca2+ Depletion from Granules Inhibits Exocytosis: A Study with Insulin-Secreting Cells. J Bio Chem. 1998;273:19002–19008. doi: 10.1074/jbc.273.30.19002. [DOI] [PubMed] [Google Scholar]
- 5.Fossier P, et al. Control of the Calcium Concentration Involved in Acetylcholine Release and its Facilitation: an Additional Role for Synaptic Vesicles? Neurosci. 1998;85:85–91. doi: 10.1016/s0306-4522(97)00591-5. [DOI] [PubMed] [Google Scholar]
- 6.Rossier MF, Putney JW., Jr The Identity of the Calcium-Storing, Inositol 1,4,5—Trisphosphate-Sensitive Organelle in Non-Muscle Cells: Calciosome, Endoplasmic Reticulum … or Both? Tr Neurosci. 1991;14:310–314. doi: 10.1016/0166-2236(91)90143-i. [DOI] [PubMed] [Google Scholar]
- 7.Verkhratsky A, Toescu EC. Endoplasmic Reticulum Ca2+ Homeostatis and Neuronal Death. J Cell Mol Med. 2003;7:351–361. doi: 10.1111/j.1582-4934.2003.tb00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang G, Holz GG. Amplification of Exocytosis by Ca2+-Induced Ca2+ Release in INS-1 Pancreatic β Cells. J Phys. 2003;546:175–189. doi: 10.1113/jphysiol.2002.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Videen JS, Mezger MS, Chang YM, O'Connor DT. Calcium and Catecholamine Interactions with Adrenal Chromogranins. Comparison of Driving Forces in Binding and Aggregation. J Bio Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 10.Yoo SH, Lewis MS. Effects of pH and Ca2+ on Monomer-Dimer and Monomer-Tetramer Equilibria of Chromogranin A. J Bio Chem. 1992;267:11236–11241. [PubMed] [Google Scholar]
- 11.Haigh JR, Parris R, Phillips JH. Free Concentrations of Sodium, Potassium, and Calcium in Chromaffin Granules. Biochem J. 1989;259:485–491. doi: 10.1042/bj2590485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahapatra NR, et al. A Dynamic Pool of Calcium in Catecholamine Storage Vesicles. J Bio Chem. 2004;279:51107–51121. doi: 10.1074/jbc.M408742200. [DOI] [PubMed] [Google Scholar]
- 13.Mundorf ML, Hochstetler SE, Wightman RM. Amine Weak Bases Disrupt Vesicular Storage and Promote Exocytosis in Chromaffin Cells. J Neurochem. 1999;73:2397–2405. doi: 10.1046/j.1471-4159.1999.0732397.x. [DOI] [PubMed] [Google Scholar]
- 14.Mundorf ML, Troyer KP, Hochstetler SE, Near JA, Wightman RM. Vesicular Ca2+ Participates in the Catalysis of Exocytosis. The Journal of Biological Chemistry. 2000;275:9136–9142. doi: 10.1074/jbc.275.13.9136. [DOI] [PubMed] [Google Scholar]
- 15.Moreno A, et al. Calcium Dynamics in Catecholamine-Containing Secretory Vesicles. Cell Cal. 2005;37:555–564. doi: 10.1016/j.ceca.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell KJ, et al. Dense Core Secretory Vesicles Revealed as a Dynamic Ca2+ Store in Neuroendocrine Cells with a Vescile-Associated Membrane Protein Aequorin Chimaera. Journal of Cell Biology. 2001;155:45–51. doi: 10.1083/jcb.200103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cano Abad MF, Di Benedetto G, Magalhaes PJ, Filippin L, Pozzan T. Mitochondrial pH Monitored by a New Engineered Green Fluorescent Protein Mutant. J Bio Chem. 2004;279:11521–11529. doi: 10.1074/jbc.M306766200. [DOI] [PubMed] [Google Scholar]
- 18.Yang DM, Kao LS. Relative Contribution of the Na+/Ca2+ Exchanger, Mitochrondria and Endoplasmic Reticulum in the Regulation of Cytosolic Ca2+ amd Catecholamine Secretion of Bovine Adrenal Chromaffin Cells. J Neurochem. 2001;76:210–216. doi: 10.1046/j.1471-4159.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JD, Chang JP. Calcium Buffering Activity of Mitochondria Controls Basal Growth Hormone Secretion and Modulates Specific Neuropeptide Signaling. Cell Cal. 2005;37:573–581. doi: 10.1016/j.ceca.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Xu T, Naraghi M, Kang H, Neher E. Kinetic Studies of Ca2+ Binding and Ca2+ Clearance in the Cytosol of Adrenal Chromaffin Cells. Biophys J. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrington J, Park YB, Babcock DF, Hille B. Dominant Role of Mitochondria in Clearance of Large Ca2+ Loads from Rat Adrenal Chromaffin Cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 22.Sturdik E, et al. Relationships Among Structure, Reactivity Towards Thiols, adn Basicity of Phenylhydrazonopropanedinitriles Collection of Czechoslovak. Chemical Communications. 1985;50:2065–2076. [Google Scholar]
- 23.Finnegan JM, Borges R, Wightman RM. Comparison of Cytosolic Ca2+ and Exocytosis Responses from Single Rat and Bovine Chromaffin Cells. Neurosci. 1996;71:833–43. doi: 10.1016/0306-4522(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 24.Park YB, Herrington J, Babcock DF, Hille B. Ca2+ Clearance Mechanisms in Isolated Rat Adrenal Chromaffin Cells. J Phys. 1996;492:329–346. doi: 10.1113/jphysiol.1996.sp021312. [DOI] [PMC free article] [PubMed] [Google Scholar]


