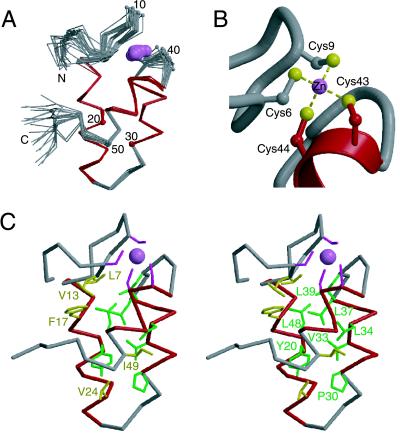
Figure 2.
NMR-derived zinc-bundle structure of mtRPB10. (A) The α-trace of 20 structures of mtRBP10, superimposed by using the main chain helical atoms (14–26, 30–37, and 42–48). The helices are highlighted in red, and the zinc ion is shown as a pink ball. Every tenth residue is numbered. The N- and C-terminal residues Met1, Ile2, Glu53, Thr54, and Trp55 are conformationally flexible in solution, as evident by heteronuclear 1H{15N} NOE values less than 0.5. (B) Zinc binding site in mtRPB10. (C) Stereo view of a representative mtRPB10 structure displaying residues within the hydrophobic core: Leu7 and Val13 in the N-terminal region, Phe17, Tyr20, and Val24 of helix 1, Pro30, Val33, Leu34, and Leu37 of helix 2, Leu39 in the loop between helix 2 and 3, Leu48, Ile49 of helix 3, and Val52 in the C-terminal region. These conserved residues are shown according to the color scheme of Fig. 1.