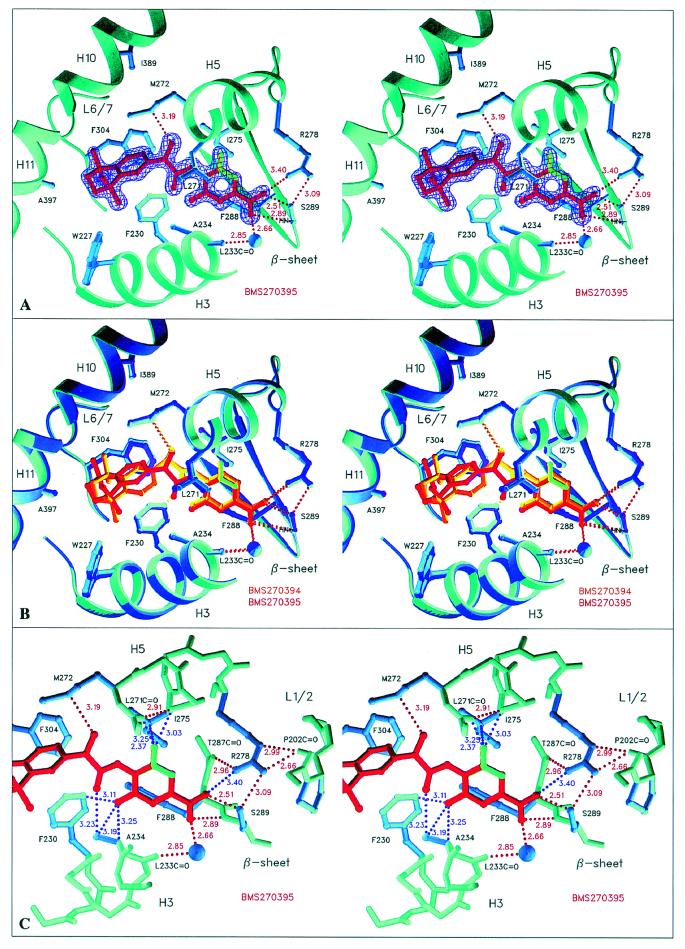
Figure 2.
Structure of hRARγ LBD bound to the inactive BMS270395 and its comparison with the BMS270394 complex (stereo representations) (A). The BMS270395 complex with the initial refinement-unbiased σA-weighted Fobs − Fcalc map at 1.67 Å resolution contoured at 3.2 σ and colored in violet. The map clearly indicates two possible positions for the fluorine atom, corresponding to two different ligand conformations. The up and down orientations of the fluorine atom have occupancies of 40/60%, respectively (colored in green and red, pointing to Ile-275 and Ala-234, respectively) (B). Superposition of the hRARγ LBD complexes of both enantiomers as obtained by a least-squares fit. The position of the hydroxyl group oxygen is strictly conserved to maintain the hydrogen bond to Met-272; BMS270395 therefore adopts a conformation different from that observed for BMS270394 (C). Detailed view of the part of the ligand pocket where the ligand exhibits unfavorable contacts. The color code for distances is that of Fig. 1. The fluorine atom exhibits close contacts for both the up and down orientation, whereas the salt bridge between the carboxylate group and Arg-278 is weaker compared with the BMS270394 complex.