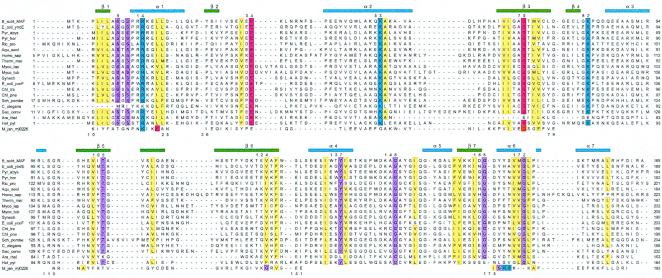
Figure 2.
Sequence alignment of the Maf protein from B. subtilis (Upper) and homologous proteins from 18 selected archaea, prokaryotes, and eukaryotes. The sequence of Mj0226 from M. jannaschii is shown (Lower); gaps in its sequence indicate regions with larger deviations between the Maf and Mj0226 structures, preventing meaningful structure-based alignment. Conserved residues are highlighted (blue, basic; red, acidic; yellow, hydrophobic; magenta, all others), secondary structure elements observed in the Maf crystal structure are indicated (Upper), and selected conserved residues in Maf are numbered.