Abstract
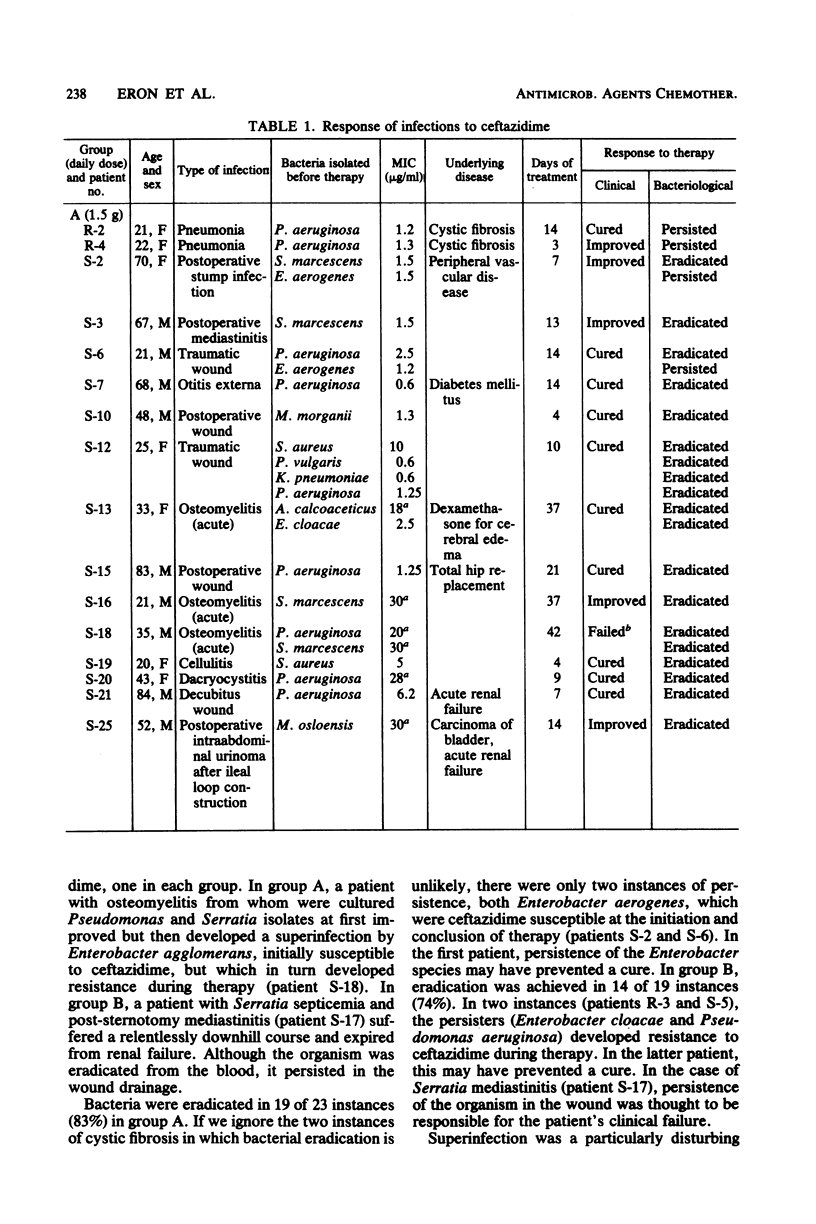
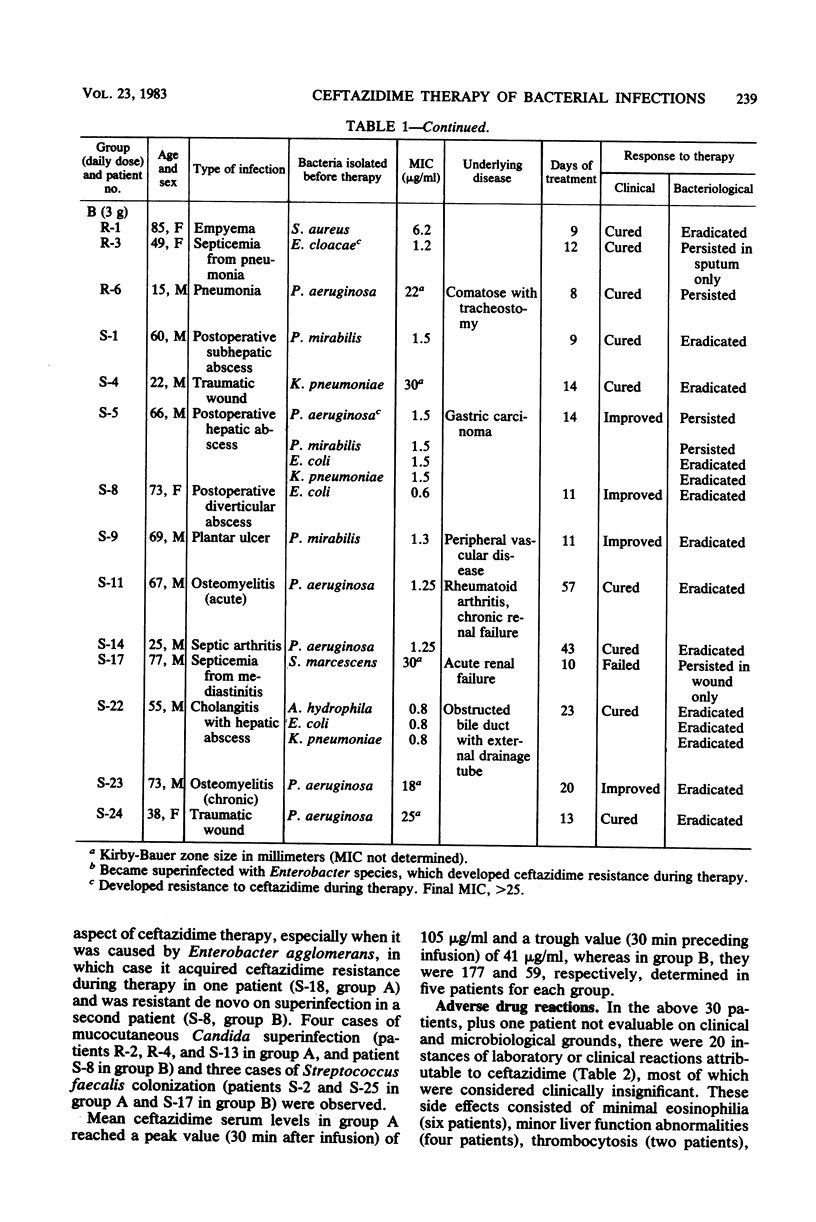
Ceftazidime, a new broad-spectrum cephalosporin, was administered to 30 patients with serious bacterial infections in a randomized dosing trial with daily doses of 1.5 or 3 g. Both regimens were equally efficacious, with satisfactory clinical responses in 28 instances (93%) and microbiological eradication of 79% of initial bacterial isolates. The development of resistance to ceftazidime during therapy was observed in three cases (Enterobacter agglomerans, Enterobacter cloacae, and Pseudomonas aeruginosa) and superinfection by a resistant Enterobacter agglomerans strain occurred in one case. Adverse reactions of clinical significance included one case each of leukopenia, azotemia, diarrhea (Clostridium difficile toxin positive), and rash.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzard D. I., Geddes A. M., Farrell I. D., Eykyn S. J., Phillips I., Wise R., Brown R. M. Ceftazidime--a new extended-spectrum cephalosporin. Lancet. 1982 May 22;1(8282):1152–1156. doi: 10.1016/s0140-6736(82)92228-0. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Antibacterial activity and beta-lactamase stability of ceftazidime, an aminothiazolyl cephalosporin potentially active against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Jan;21(1):11–18. doi: 10.1128/aac.21.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Acred P., Harper P. B., Ryan D. M., Kirby S. M., Harding S. M. GR 20263, a new broad-spectrum cephalosporin with anti-pseudomonal activity. Antimicrob Agents Chemother. 1980 May;17(5):876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt R., Ehrlich S. L., Afarian J., O'Brien T. F., Pennington J. E., Kass E. H. Moxalactam therapy of infections caused by cephalothin-resistant bacteria: influence of serum inhibitory activity on clinical response and acquisition of antibiotic resistance during therapy. Antimicrob Agents Chemother. 1981 Sep;20(3):351–355. doi: 10.1128/aac.20.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]


