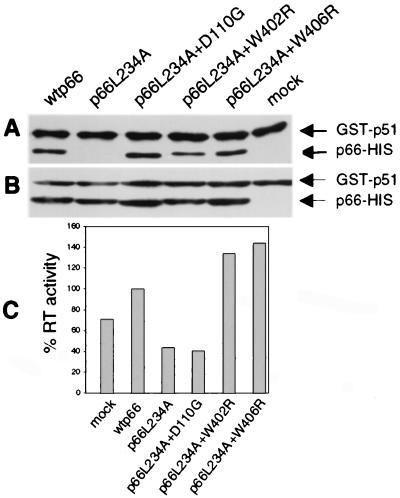
Figure 6.
In vitro assay for binding of GST-p51 and p66 to form active RT heterodimers. (A) Bacterial lysates containing GST-p51 and various p66 proteins as indicated were incubated overnight and were captured on glutathione beads. The complexes were eluted, were resolved by SDS/PAGE, were blotted to membrane, and were detected by monoclonal anti-RT antibodies. Mock, GST-p51 alone. (B) An aliquot of each incubation mix, reflecting input protein, was directly analyzed by SDS/PAGE and Western blot analysis as in A. (C) Bound proteins were eluted with glutathione and were assayed for RT activity with homopolymeric template-primer. Values are normalized to the wild-type control.