Abstract
Background
Patients with common variable immunodeficiency may exhibit rapidly progressive hepatitis when infected with hepatitis C virus (HCV), leading to cirrhosis and liver failure. Liver transplantation in these patients may result in a cholestatic form of HCV reinfection with exceptionally high virus loads.
Aims
To report an immunohistochemical investigation of the pretransplant and post‐transplant liver of one such patient.
Methods/Results
On immunohistochemical staining of frozen sections with anti‐HCV core monoclonal antibody or fluorescein labelled human polyclonal anti‐HCV IgG, no HCV antigens were demonstrated in the native cirrhotic liver removed at transplant, despite a viral load of 106.4 genomes/g. The transplanted liver, collected six weeks post‐transplant, exhibited cholestatic recurrent hepatitis, had an HCV virus load of 1010 genomes/g of liver, and revealed HCV antigen in the cytoplasm of most hepatocytes, with a pronounced periportal distribution. No virus antigen was demonstrable in other cell types. The core antigen was also detected in paraffin wax embedded, formaldehyde fixed tissue of this liver after high temperature antigen retrieval, but not in the native cirrhotic liver or a selection of HCV positive livers collected pretransplant from immunocompetent patients. Attempts to delineate the distribution of E1, NS3, and NS4 antigens were unsuccessful because monoclonal antibodies to these antigens produced “false positive” staining of foci of hepatocytes in the post‐transplant livers of HCV seronegative patients with cholestasis.
Conclusion
This case provided an opportunity to study the natural development of HCV during acute infection in the absence of an immune response, and may help to elucidate the pathogenesis of HCV recurrence in liver allografts.
Keywords: common variable immunodeficiency, monoclonal antibody
Orthotopic liver transplantation of patients with hepatitis C virus (HCV) associated liver failure has been relatively successful in the short term, but recurrence of HCV infection in the grafted liver is inevitable and HCV infection impairs patient and allograft survival.1 On recurrence, concentrations of HCV RNA in the serum may reach 10–20 times pre‐transplantation values,2 and high antigen expression in the transplanted liver is associated with the development of acute and chronic hepatitis.3 In a small proportion of cases recurrence is associated with a rapidly progressive, cholestatic form of hepatitis,4 which has also been seen after living donor liver transplantation.5,6
“Although reports are few, transplantation in patients with hepatitis C virus and hypogammaglobulinaemia has generally not been successful, with a high incidence of severe cholestatic hepatitis and exceptionally high virus loads”
Patients with congenital or acquired immunodeficiencies frequently suffer a more aggressive form of HCV than immunocompetent patients, with cirrhosis and liver failure occurring in less than 10 years.7,8 Those with common variable immunodeficiency (CVID), where hypogammaglobulinaemia may be combined with T cell defects, are particularly susceptible. Although reports are few, transplantation in patients with HCV and hypogammaglobulinaemia has generally not been successful, with a high incidence of severe cholestatic hepatitis and exceptionally high virus loads.9,10,11 These rare cases provide a valuable opportunity for studying the natural development of the virus during acute infection in the virtual absence of an immune response, and may help to elucidate the pathogenesis of HCV recurrence in the liver allograft.12
Here, we report a virological and immunohistochemical investigation of a case of severe cholestatic hepatitis and recurrent HCV infection post‐orthotopic liver transplantation in a patient with CVID.
Methods
Antibodies
Table 1 provides details of the monoclonal antibodies used in our study. Fluorescein conjugated polyclonal human anti‐HCV IgG antibody was kindly provided by Dr G Ballardini, University of Bologna, Italy.13 Optimal dilutions of all antibody preparations were determined by titration, either on pellets of HepG2 cells infected with vaccinia recombinant viruses expressing the structural protein genes of a type 1a strain of HCV,14 or on sections of liver S2 (see below) fixed and treated by the appropriate method.
Table 1 Monoclonal antibodies used.
| MAb | Specificity | Isotype | Source |
|---|---|---|---|
| MAb φ126 | HCV core | IgG2a | Biogenesis, Poole, UK |
| 11B7D8 | HCV E1 | Unknown | Dr G Maertens, Innogenetics, Ghent, Belgium |
| 6A3.2 | HCV NS3 | IgG2b | Novocastra Laboratories, Newcastle upon Tyne, UK |
| Tordji‐22 | HCV NS4 | IgG1 | Biomen Ltd, Finchampstead, Berkshire, UK |
| 10/5.1.2 | Adenovirus | IgG2a | Novocastra Laboratories |
| BA‐4 | Elastin | IgG1 | Novocastra Laboratories |
HCV, hepatitis C virus; MAb, monoclonal antibody.
Patients and tissue samples
Patient S, who was diagnosed as having CVID two years before presentation with acute HCV infection, believed to result from receiving infected Gammagard (genotype 1a), has been described previously.14 She required orthotopic liver transplantation for severe HCV related cirrhosis and liver failure. After transplantation, her liver function deteriorated and she was re‐transplanted six weeks after her first operation. Samples of explant liver and serum were collected at each transplant (SI and S2) and stored at −70°C, with the patient's informed consent.
Explant liver samples from a further 12 patients at transplant for end stage liver disease and transplant livers from three patients at retransplant were collected for comparison (table 2). HCV positive patients were seropositive by both ELISA‐2 (Abbott Laboratories; United Biomedical, Hauppage, New York, USA) and RIBA‐2 (Ortho Diagnostic Systems, Raritan, New Jersey, USA), HCV RNA positive by reverse transcription polymerase chain reaction (RT‐PCR), and infected with HCV genotype 1a (one) or 1b (three). HCV negative patients were seronegative.
Table 2 Summary of immunohistochemistry for HCV antigens in transplant and non‐transplant liver tissue.
| Tissue sample | HCV status | Fixation | Human polyclonal antibody | MAb φ126 (core) | MAb 11B7D8 (E1) | MAb 6A3.2 (NS3) | MAb Tordji‐22 (NS4) |
|---|---|---|---|---|---|---|---|
| Explant livers | |||||||
| S1 | + (1a) | P | ND | − | − | − | − |
| F | − | − | − | − | − | ||
| A | + (1a) | F | − | − | − | − | − |
| B | + (1b) | F | + | − | − | − | − |
| C | + (1b) | F | − | − | − | − | − |
| D | + (1b) | F | − | − | − | − | − |
| E | − | F | − | − | − | − | − |
| F | − | F | − | − | − | − | − |
| G | − | F | − | − | − | − | − |
| H | − | F | − | − | − | − | − |
| I | − | F | − | − | − | − | − |
| J | − | F | − | − | − | − | − |
| Transplant livers with cholestatic liver disease | |||||||
| S2 | + (1a) | P | ND | + | + | + | + |
| F | + | + | + | + | + | ||
| K | − | P | ND | − | + | + | + |
| L | − | P | ND | − | + | + | + |
| M | − | F | − | − | − | − | − |
| Explant livers with cholestatic liver disease | |||||||
| N | − | P | ND | − | − | − | − |
| O | − | P | ND | − | − | − | − |
F, frozen; MAb, monoclonal antibody; ND, not done; P, formalin fixed and paraffin wax embedded.
Routine formaldehyde fixed and paraffin wax embedded sections of livers were selected from the files of the department of cellular pathology within the Newcastle upon Tyne NHS Trust, UK.
Quantitative PCR on liver and serum
Liver samples were macerated and the HCV RNA content of liver and serum was evaluated by quantitative RT‐PCR as described previously.15
Immunostaining
Cubes (1 cm) of fresh explant liver were coated in OCT and frozen in isopropyl alcohol cooled in liquid nitrogen. Cryostat sections (5–6 nm) were air dried, fixed in 1/1 acetone/chloroform at room temperature, and stored at −70°C. Alternatively, cubes of fresh tissue were soaked in 4% paraformaldehyde in 30% sucrose until saturated. The fixed tissue was then frozen in OCT, sectioned, and dried as above but stored without further fixation. Sections (5 μm) were cut from percutaneous needle liver biopsies or postmortem tissue blocks, fixed in formalin, and embedded in paraffin wax.
Sections were subjected to high temperature antigen unmasking where appropriate and stained with monoclonal antibodies as described by Bevitt et al.16 Specific antibody staining was visualised either by the five step peroxidase antiperoxidase protocol of Gonzalez‐Peralta et al,17 or by the En‐Vision method (Dako Cytomation, Ely, Cambridgeshire, UK) according to the manufacturer's instructions.
Frozen sections only were stained with human polyclonal anti‐HCV IgG by the method described by Ballardini et al,13 without prior quenching of endogenous peroxidase.
Blocking of monoclonal antibody binding with recombinant core protein
A 10 μg/ml solution of monoclonal antibody φ126 in Tris buffered saline was mixed with an equal volume of a 10 μg/ml solution of recombinant polyhistidine tagged HCV core protein18 or recombinant polyhistidine tagged human cytomegalovirus pp65 antigen purified by affinity chromatography on nickel‐sepharose (Novocastra Laboratories, Newcastle upon Tyne, UK). The mixtures were incubated at room temperature for 30 minutes before staining sections of HCV positive and negative liver.
Results
Histological and virological evaluation of liver S2
Explant liver S2 was collected at retransplant six weeks after primary transplantation into a patient suffering from CVID and chronic HCV infection. Histology of S2 showed a moderate hepatitis with inflammation in portal tracts and foci of interface hepatitis; lobular inflammation was also present. There was pronounced bilirubinostasis with cholestatic rosette formation. There was also evidence of perivenular injury, thought to be in part ischaemic, and some features of a classic acute cellular rejection, with a mixed infiltrate of inflammatory cells in the portal tracts, including blast cells and eosinophils. However, the neutrophil infiltrate within the bile ducts was very pronounced, suggesting concomitant acute cellular rejection and ascending cholangitis. Overall, the findings were considered to represent cholestatic recurrent HCV infection with superimposed ischaemia secondary to hepatic artery occlusion.
Tissue macerates of S2 and the native explant cirrhotic liver (S1) and serum samples, collected immediately before the first and second liver transplant, were titrated for HCV RNA by quantitative RT‐PCR. The mean HCV RNA titre in the serum increased 30‐fold between transplants, from 106 IU/ml to 107.5 IU/ml, and the titres in the transplant liver (1010 IU/g) were almost 4000‐fold higher than those in the native liver (106.4 IU/g).
Detection of HCV antigens in frozen liver sections
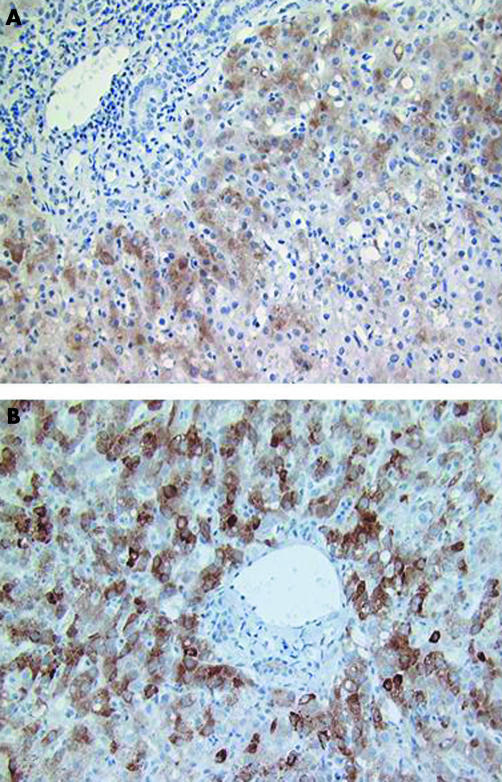
Frozen sections from livers S1 and S2, positive and negative controls (A–J and M; table 2) were stained with monoclonal antibody to HCV core protein (φ126) by the indirect method or with affinity purified human anti‐HCV polyclonal antibodies using the technique of Ballardini et al.13 With both reagents the transplant liver S2 exhibited heavy cytoplasmic staining of most of the hepatocytes, with a pronounced periportal distribution (fig 1). Specific staining was not apparent in other cell types. A similar picture was seen in both chloroform/acetone fixed sections and in paraformaldehyde fixed sections, but only after high temperature antigen unmasking of the paraformaldehyde fixed sections. With the monoclonal antibody, no specific hepatocyte staining was observed in the native liver of the same patient (S1) removed at transplant, in any of the explant livers (A–J; table 2) from HCV positive or negative patients, or in the HCV negative, heavily cholestatic transplant liver (M). Specific hepatocyte staining with the polyclonal antibody was not present in the native liver S1 and was seen in only one of four other HCV positive explant livers tested. Hepatocyte staining with monoclonal antibody φ126 in S2 was abrogated by previous absorption of the monoclonal antibody with recombinant HCV core protein expressed from Escherichia coli, but not with control recombinant cytomegalovirus pp65 antigen, and was much reduced by the absorption of cells with a lysate of HepG2 cells transiently expressing core antigen from vaccinia virus, but not with lysates of HepG2 infected with wild‐type vaccinia virus (data not shown). A monoclonal antibody of the same isotype as φ126 but of irrelevant specificity showed no staining of hepatocytes in the liver sections. Monoclonal antibody φ126 also produced a distinctive pattern of staining of connective tissue in liver of all patients and in liver, skin, and muscle of HCV negative patients, similar to that seen when these tissues were stained with anti‐elastin monoclonal antibody (NCL‐elastin). This distinctive pattern of non‐specific staining was easily differentiated from specific HCV staining in hepatocytes.
Figure 1 Paraformaldehyde fixed, frozen sections of post‐transplant liver S2. (A) Stained with fluorescein labelled human polyclonal anti‐hepatitis C virus IgG according to the method of Ballardini et al.13 (B) Stained with anti‐core monoclonal antibody φ126 using the five step peroxidase antiperoxidase method.
Evaluation of φ 126 on formaldehyde fixed, paraffin wax embedded sections
Formaldehyde fixed, paraffin wax embedded sections of livers S1 and S2 were stained with monoclonal antibody φ126 (anti‐core). No specific staining of hepatocytes was seen in liver S1, but cytoplasmic staining of hepatocytes was seen in S2 after high temperature antigen unmasking only, showing that the core protein epitope is stable during routine processing for immunohistochemistry. A monoclonal antibody of irrelevant specificity but matched for immunoglobulin isotype produced no staining of S2. Sections from four HCV seronegative patients also exhibiting cholestasis (K, L, N, and O), two of which were collected post‐transplantation, and slides from a further seven archival HCV positive cases and seven HCV negative cases (not shown) were stained with monoclonal antibody φ126. No unambiguous staining of hepatocytes was seen in these sections.
Evaluation of additional monoclonal antibodies
The composition of the HCV antigens demonstrated in S2 hepatocytes was also probed with monoclonal antibodies to the E1 and NS3 antigens previously shown to react with the antigen in S2 liver by western blotting,14 and with the NS4 specific Tordji‐22 monoclonal antibody, which has previously been evaluated in immunohistochemistry (table 1).19,20 All three additional monoclonal antibodies produced intense cytoplasmic staining of hepatocytes, similar to that seen with monoclonal antibody φ126. However, unlike φ126, all three monoclonal antibodies also stained small foci of hepatocytes in the paraffin wax embedded livers of cholestatic HCV negative patients collected post‐transplant.
Discussion
Six weeks after transplantation, patient S, who had previously shown an exceptional sensitivity to HCV infection associated with CVID, exhibited recurrent HCV infection with severe cholestatic hepatitis. In keeping with the exceptionally high virus loads in both liver and serum, we found heavy cytoplasmic expression of core antigen in hepatocytes throughout the liver at this time, with a pronounced periportal distribution. Hepatocytes were the only cell type expressing viral antigens.
Specific hepatocyte staining of HCV core with monoclonal antibody φ126 was seen in both frozen and paraffin wax embedded sections of liver S2. Unlike Tordji‐22, which has previously been evaluated for HCV staining of paraffin wax embedded tissue sections,20 and several other anti‐HCV monoclonal antibodies tested here, φ126 did not crossreact with non‐HCV antigens in the hepatocytes of cholestatic, post‐transplant liver. However, it is noteworthy that φ126 did react with a host antigen, codistributed with elastin, although this was present in all sections of liver and in other tissues, and was clearly differentiated from HCV specific staining. Antigenic mimicry by HCV has been reported previously,21,22 and may play a role in viral pathogenesis.
“Our results underline the care that is required in the interpretation of immunohistochemical studies on hepatitis C virus, which may share epitopes with host and other viral antigens”
Immunohistochemistry has generally been found to have insufficient sensitivity for the routine demonstration of viral antigens in the livers of patients chronically infected with HCV.23,24 However, Ballardini et al have demonstrated HCV antigens in the livers of over 80% of persistently infected patients stained with human polyclonal antiserum.25 In our hands, both Ballardini's antiserum and monoclonal antibody φ126 had a somewhat lower sensitivity. Although virus antigen expression was demonstrable in S2, positively stained cells were not unambiguously identified in frozen sections of liver S1 with a 4000‐fold lower viral load, or in three of four HCV positive explant livers from immunocompetent patients or routinely processed HCV positive sections randomly drawn from the archives.
Take home messages
We describe a rare case of common variable immunodeficiency in which the patient exhibited rapidly progressive hepatitis when infected with hepatitis C virus (HCV), leading to cirrhosis and liver failure; liver transplantation resulted in a cholestatic form of HCV reinfection with exceptionally high virus loads
Immunohistochemical staining with anti‐HCV core monoclonal antibody or polyclonal anti‐HCV IgG was negative in the native cirrhotic liver removed at transplant, despite a viral load of 106.4 genomes/g
The transplanted liver, collected six weeks post‐transplant, exhibited cholestatic recurrent hepatitis, had an HCV virus load of 1010 genomes/g of liver, and revealed HCV antigen in the cytoplasm of most hepatocytes, with a pronounced periportal distribution
Attempts to delineate the distribution of E1, NS3, and NS4 antigens were unsuccessful because monoclonal antibodies to these antigens produced “false positive” staining of foci of hepatocytes in the post‐transplant livers of HCV seronegative patients with cholestasis
Because adequate cell culture systems for the virus have proved difficult to establish, the demonstration of high viral antigen expression in liver S2 provides a valuable opportunity to study the natural development of the virus during acute infection in the virtual absence of an immune response, and may help to elucidate the pathogenesis of HCV recurrence in the liver allograft. Further immunohistochemical evaluation of material of this type may also yield valuable insights into the morphogenesis of virions not available currently from cell culture studies. However, our results underline the care that is required in the interpretation of immunohistochemical studies on HCV, which may share epitopes with host and other viral antigens.
Abbreviations
CVID - common variable immunodeficiency
HCV - hepatitis C virus
RT‐PCR - reverse transcription polymerase chain reaction
References
- 1.Forman L, Lewis J, Berlin J.et al The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 20021221162–1165. [DOI] [PubMed] [Google Scholar]
- 2.Gane E, Naoumov N, Qian K‐P.et al A longitudinal analysis of hepatitis C virus replication following liver transplantation. Gastroenterology 1996110167–177. [DOI] [PubMed] [Google Scholar]
- 3.Vargas V, Krawczynski K, Castells L.et al Recurrent hepatitis C virus infection after liver transplantation: immunohistochemical assessment of the viral antigen. Liver Transpl Surg 19984320–327. [DOI] [PubMed] [Google Scholar]
- 4.Schluger L, Sheiner P, Thung S.et al Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 199623971–976. [DOI] [PubMed] [Google Scholar]
- 5.Troppmann C, Rossaro L, Perez R.et al Early, rapidly progressive cholestatic hepatitis C reinfection and graft loss after adult living donor liver transplantation. Transplantation 20033239–240. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko J, Sugawara Y, Akamatsu N.et al Cholestatic hepatitis due to hepatitis C virus after a living donor liver transplantation. Hepatogastroenterology 200451243–244. [PubMed] [Google Scholar]
- 7.Healey C, Sabharwal N, Daub J.et al Outbreak of acute hepatitis C following the use of anti‐hepatitis C virus‐screened intravenous immunoglobulin therapy. Gastroenterology 19961101120–1126. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez S, Talal A. Hepatitis virus in human immunodeficiency virus‐infected individuals: an emerging comorbidity with significant implications. Semin Liver Dis 200323149–166. [DOI] [PubMed] [Google Scholar]
- 9.Bjoro K, Schrumpf E, Bergan A.et al Liver transplantation for endstage hepatitis C cirrhosis in a patient with primary hypogammaglobulinaemia. Scand J Infect Dis 199830520–522. [DOI] [PubMed] [Google Scholar]
- 10.Smith M, Webster A, Dhillon A.et al Orthotopic liver‐transplantation for chronic hepatitis in patients with common variable immunodeficiency. Gastroenterology 1995108879–884. [DOI] [PubMed] [Google Scholar]
- 11.Gow P, Mutimer D. Successful outcome of liver transplantation in a patient with hepatitis C and common variable immune deficiency. Transpl Int 200215380–383. [DOI] [PubMed] [Google Scholar]
- 12.McCaughan G, Zekry A. Pathogenesis of hepatitis C virus recurrence in the liver allograft. Liver Transpl 20028S7–13. [DOI] [PubMed] [Google Scholar]
- 13.Ballardini G, Groff P, Giostra F.et al Hepatocellular codistribution of c100, c33, c22, and NS5 hepatitis C virus antigens detected using immunopurified polyclonal spontaneous human antibodies. Hepatology 199521730–734. [PubMed] [Google Scholar]
- 14.Nielsen S, Bassendine M, Burt A.et al Characterisation of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J Gen Virol 2004851487–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pumeechockchai W, Bevitt D, Agarwal K.et al Hepatitis C virus particles of different density in the blood of chronically infected immunocompetent and immunodeficient patients: implications for virus clearance. J Med Virol 200268335–342. [DOI] [PubMed] [Google Scholar]
- 16.Bevitt D J, Milton I D, Piggot N.et al New monoclonal antibodies to oestrogen and progesterone receptors effective for paraffin section immunohistochemistry. J Pathol 1997183228–232. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez‐Peralta R, Fang J, Davis G.et al Optimization for the detection of hepatitis C virus antigens in the liver. J Hepatol 199420143–147. [DOI] [PubMed] [Google Scholar]
- 18.Milton I, Watson J, Guo K.et al Prokaryotic expression and analysis of the antibody response to a Newcastle isolate of the core gene of hepatitis C. J Med Virol 199545253–258. [DOI] [PubMed] [Google Scholar]
- 19.Brody R, Eng S, Melamed J.et al Immunohistochemical detection of hepatitis C antigen by monoclonal antibody Tordji‐22 compared with PCR viral detection. Am J Clin Pathol 199811032–37. [DOI] [PubMed] [Google Scholar]
- 20.Doughty A, Painter D, McCaughan G. Nonspecificity of monoclonal antibody Tordji‐22 for the detection of hepatitis C virus in liver transplant recipients with cholestatic hepatitis. Liver Transpl Surg 1999540–45. [DOI] [PubMed] [Google Scholar]
- 21.Gregorio G V, Pensati P, Iorio R.et al Autoantibody prevalence in children with liver disease due to chronic hepatitis C virus (HCV) infection. Clin Exp Immunol 1998112471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroga J A, Pardo M, Navas S.et al Patterns of immune responses to the host‐encoded GOR and hepatitis C virus core‐derived epitopes with relation to hepatitis C viremia, genotypes, and liver disease severity. J Infect Dis 1996173300–305. [DOI] [PubMed] [Google Scholar]
- 23.Lau J, Krawcynski K, Negro F.et al In situ detection of hepatitis C virus—a critical appraisal. J Hepatol 199624(suppl 2)43–51. [PubMed] [Google Scholar]
- 24.Scheuer P, Krawczynski K, Dhillon A. Histopathology and detection of hepatitis C virus in liver. Springer Semin Immunopathol 19971927–45. [DOI] [PubMed] [Google Scholar]
- 25.Ballardini G, Groff P, Pontisso P.et al Hepatitis C virus (HCV) genotype, tissue HCV antigens, hepatocellular expression of HLA‐A,B,C, and intracellular adhesion‐molecules. J Clin Invest 1995952067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]