Abstract
Aims
Compact tryptase‐positive round cell infiltrates of the bone marrow (TROCI‐BM) are very rare histopathological findings and may pose challenging problems with regard to the cell type involved (either mast cells or basophilic granulocytes) and the exact diagnosis.
Methods
A selected panel of immunohistochemical markers against mast cell and basophil related antigens, including CD25, CD34, CD117/Kit, and the 2D7 antigen (which is found only in basophilic granulocytes) on a total of 410 routinely processed bone marrow biopsy specimens (including 88 cases of systemic mastocytosis (SM), 20 cases of chronic myeloid leukaemia (CML), 92 cases of myeloid neoplasms other than CML, and 210 controls with normal/reactive bone marrows).
Results
In total, 17 cases with TROCI‐BM could be identified: 11 SM (including two cases of well‐differentiated SM and two mast cell leukaemias; MCL), 2 myelomastocytic leukaemia (MML), 2 CML with excess of basophils (secondary basophilic leukaemia (CMLba)), and 2 tryptase positive acute myeloid leukaemia (AML). Regarding the cell types involved, TROCI‐BM cells were found to express CD117/Kit in all cases of SM and MCL. In MML and tryptase postitive AML, TROCI‐BM cells were found to coexpress CD34 and Kit. The basophil specific antigen 2D7 was only detected in CD34/Kit negative TROCI‐BM cells in two patients with CMLba. The activating point mutation D816V was detected in 8/11 patients with SM but not in any of the other haematological malignancies.
Conclusions
In summary, a total of six rare myeloid neoplasms may present with a novel immunohistochemical phenomenon tentatively termed TROCI‐BM.
Keywords: systemic mastocytosis, basophilic leukaemia, mast cell leukaemia, tryptase, chronic myeloid leukaemia, myelomastocytic leukaemia
The application of tryptase antibodies as immunohistochemical markers in the histopathological analysis of routinely processed bone marrow (BM) biopsy specimens is of crucial importance for the diagnosis of systemic mastocytosis (SM).1,2,3 It has been shown that tryptase immunohistochemistry enables not only the detection of loosely scattered mast cells (MC) but also of small compact MC infiltrates as the major diagnostic criterion for SM.4,5 A proportion of intralesional MC usually exhibits atypical cytology, including hypogranulation and spindle shape appearance, the latter being a minor diagnostic criterion thus enabling the immediate histopathological diagnosis of SM by application of the WHO classification criteria.6,7 In rare SM cases, however, virtually all intralesional tryptase positive MC are round. Such findings prompted us to look for the frequency of “round cell” SM. Focal and diffuse tryptase positive round cell infiltrates could also be detected in myeloid neoplasms other than SM but were almost never seen in normal/reactive bone marrows. The tryptase positive round cell infiltrate of the BM (tentatively referred to as TROCI‐BM) proved to be a major diagnostic challenge in haematopathology. To address this issue appropriately, we applied lineage specific markers to all cases of TROCI‐BM. The use of antibodies against CD25, CD117/Kit, and chymase appears to be sufficient to separate neoplastic MC from basophils expressing the 2D7antigen.8,9,10
MATERIALS AND METHODS
Selection of cases
Cases were derived retrospectively from the archival files of the Institutes of Pathology, Universities of Lübeck, Tübingen (both Germany), and Vienna (Austria). The selection of cases was based on the identification of a tryptase positive round cell infiltrate in the BM (TROCI‐BM). TROCI‐BM was defined as an infiltrate consisting of focal or diffuse compact aggregates of round tryptase positive cells. Primary diagnoses had been established according to WHO criteria and recommendations of the French‐American‐British (FAB) cooperative study group.11,12,13 (a) The control group comprised 210 cases with normal or reactive bone marrows, in particular with no evidence of a myeloid neoplasm or mastocytosis; (b) SM including mast cell leukaemia (MCL): morphological analysis of BM cells in 88 cases of SM revealed nine cases exhibiting focal, and two with a diffuse infiltrates featuring TROCI‐BM. In the latter two cases, the diagnosis of MCL (exhibiting >20% atypical MC in BM smears) had been established. (c) Myelomastocytic leukaemia (MML): this extremely rare myeloid neoplasm is characterised by prominent MC differentiation with increased numbers of immature metachromatic cells belonging to the MC lineage cells but without fulfilling the diagnostic criteria of SM.14,15,16 In the current analysis, two patients with MML exhibiting TROCI‐BM were included. (d) Tryptase positive acute myeloid leukaemia (AML): in a small subgroup of patients with AML, blast cells expressed immunohistochemically detectable levels of tryptase without any visible metachromatic granules (thereby clearly contrasting to MML).17,18 In the present study, we identified only two cases of tryptase postitive AML, belonging to subtypes M0 and M1, respectively, according to FAB criteria.12 None of the analysed cases of myelodysplastic and myeloproliferative syndromes or of myelodysplastic/myeloproliferative syndromes exhibited TROCI‐BM. (e) Chronic myeloid leukaemia (CML): a survey of 20 cases of CML revealed two (10%) with TROCI‐BM. Both patients were found to be clinically and morphologically in the accelerated phase of their disease (CML‐AP).
The main clinical and biological characteristics of the patients are summarised in table 1.
Table 1 Tryptase positive round cell infiltrates in the bone marrow (TROCI‐BM): list of diagnoses and main biological features of the patients.
| Case no. | Age (years) | Sex | Diagnosis (number of focal TROCI‐BM) | CD25 | D816V | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26 | F | Systemicmastocytosis* (n = 4) | Negative | Negative | |||||
| 2 | 63 | M | Systemic mastocytosis* (n = 7) | Negative | Negative | |||||
| 3 | 47 | F | Systemic mastocytosis (n = 4) | Positive | Positive | |||||
| 4 | 87 | F | Systemic mastocytosis (n = 2) | Positive | Positive | |||||
| 5 | 51 | F | Systemic mastocytosis (n = 3) | Positive | Positive | |||||
| 6 | 36 | M | Systemic mastocytosis (n = 2) | Positive | Positive | |||||
| 7 | 53 | F | Systemic mastocytosis (n = 3) | Positive | Positive | |||||
| 8 | 58 | F | Systemic mastocytosis (n = 2) | Positive | Positive | |||||
| 9 | 63 | M | Systemic mastocytosis (n = 7) | Positive | Positive | |||||
| 10 | 48 | M | Mast cell leukaemia (N/A) | Positive | Negative | |||||
| 11 | 75 | M | Mast cell leukaemia (N/A) | Positive | Positive | |||||
| 12 | 71 | M | Myelomastocytic leukaemia (N/A) | Negative | Negative | |||||
| 13 | 69 | M | Myelomastocytic leukaemia (N/A) | Positive | Negative | |||||
| 14 | 46 | M | Tryptase positive acute myeloid leukaemia (N/A) | Negative | Negative | |||||
| 15 | 68 | F | Tryptase positiveacute myeloid leukaemia (N/A) | Negative | Negative | |||||
| 16 | 64 | F | Secondary basophilic leukaemia (n = 3) | Negative | Negative | |||||
| 17 | 63 | M | Secondary basophilic leukaemia (n = 2) | Negative | Negative | |||||
| 18 | 43 | F | SM‐AHNMD (SM‐CML‐CP) (n = 0) | Positive | Positive |
N/A, not applicable because of diffuse TROCI‐BM. *SM with round CD25‐negative mast cells has recently been described.27 SM‐AHNMD, systemic mastocytosis with an associated clonal haematological non‐mast cell‐lineage disorder.
Immunohistochemistry
Routinely processed BM trephine biopsy specimens had been mildly decalcified in edetic acid for at least 8 hours, embedded in paraffin wax, and stained with haematoxylin and eosin, Giemsa, Gömöri's silver impregnation, and the naphthol AS‐D chloroacetate esterase. Immunohistochemistry was performed according to the ABC method using antibodies against CD25, CD34 (both Novocastra, Hamburg, Germany), CD117, myeloperoxidase, tryptase all Dako, Hamburg, Germany), and 2D7, as described previously.1,19 The basophil specific antibody 2D7 has been intensively characterised previously10 and has now been found to be applicable to routinely processed BM sections (unpublished results). For the evaluation of marker expression by TROCI‐BM, serial sections of each case were stained with these antibodies.
Molecular studies
Screening for the activating c‐kit mutation D816V in lesional MC of the bone marrow was performed using melting point analysis of nested PCR products amplified from laser dissected single tryprase positive cells, which were pooled to a total of 50 cells per PCR tube. The techniques have been described in detail elsewhere.20,21
RESULTS
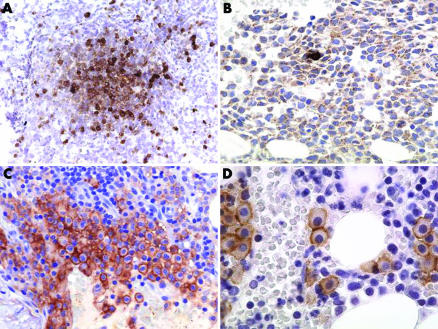
A summary of the immunophenotypic features of the neoplastic tryptase positive round cells producing TROCI‐BM is depicted in fig 1 and listed in table 2.
Figure 1 Tryptase positive round cell infiltrates of the human bone marrow (TROCI‐BM) (A) Focal TROCI‐BM stained with 2D7. The round medium sized cells expressed tryptase and the 2D7 antigen (not depicted) as clear indication of involvement of the basophil lineage in a case of accelerated phase chronic myeloid leukaemia. (B) Diffuse TROCI‐BM stained with AA1. The extremely hypercellular marrow is diffusely infiltrated by medium sized blast cells which exhibit a focal cytoplasmatic immunoreactivity for anti‐tryptase in a case of tryptase positive AML. Note the single reactive mast cells with strong expression of tryptase in the middle portion of the picture. (C) Focal TROCI‐BM stained with anti‐CD25. A compact infiltrate of round mast cells dominates the picture. Note the strong annular expression of CD25 by all the mast cells indicating an atypical immunophenotype in a case of D816V positive systemic mastocytosis. (D) Focal TROCI‐BM stained with anti‐Kit. Round mast cells forming small groups and expressing Kit (CD117) are easily detectable. The mast cells did not express CD25 not depicted, indicating the diagnosis of D816V positive well differentiated systemic mastocytosis. The ABC method was used to visualise the sections. Original magnification: (A)×25; (B, C)×140; (D)×350.
Table 2 Tryptase positive round cells in the human bone marrow: immunophenotypical differentiation.
| CD34 | CD25 | CD117 | 2D7 | Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
| − | +/−* | + | − | SM | ||||
| − | − | − | + | cML‐AP | ||||
| + | − | −/+ | − | AMLtry | ||||
| − | + | + | − | MCL | ||||
| + | − | + | − | MML |
+, Antigen is constantly expressed; +/−, antigen is expressed in a majority of cases; −/+,antigen is inconstantly expressed in a minority of patients; −, antigen is not expressed. *SM with round CD25 ‐negative round mast cells has recently been described (“well differentiated SM”). SM, systemic mastocytosis; CML‐AP, (secondary) basophilic leukaemia; AML try, tryptase positive acute myeloid leukaemia; MCL, mast cell leukaemia; MML, myelomastocytic leukaemia.
Phenotype of TROCI‐BM cells in SM and mast cell leukaemia (MCL)
Nearly 10% of all cases with SM (n = 9/88) contained focal compact BM infiltrates with a marked predominance or exclusive occurrence of tryptase expressing round cells (TROCI‐BM). Seven of these nine cases exhibited tryptase positive round cells with coexpression of CD117 and CD25, thus demonstrating the classic immunophenotype of SM. In the remaining two cases, however, TROCI‐BM cells expressed CD117, thereby indicating that they belonged to the MC lineage, but they did not express CD25. In all nine cases, TROCI‐BM did not react with the basophil specific antibody 2D7. In patients with MCL, TROCI‐BM cells were diffusely spread, thereby completely destroying the pre‐existent microarchitecture of the BM. TROCI‐BM cells expressed CD25 and CD117 but did not express CD34, an antigen that was found to be expressed on blast cells in MML and tryptase postitive AML.
Phenotype of TROCI‐BM cells in MML and tryptase postitive AML
In all four patients with tryptase postitive AML (n = 2) and MML (n = 2), TROCI‐BM was found to exhibit a diffuse pattern (fig 1). In AML, TROCI‐BM was found to consist of non‐granulated blast cells, whereas in MML, there was a mixture of metachromatic blast cells, non‐granulated blast cells, and immature MC. In both disease variants, cells of TROCI‐BM did not react with antibodies against the 2D7 antigen and CD25, but most were found to coexpress CD34.
Phenotype of TROCI‐BM cells in patients with CML and basophilia
A few scattered TROCI‐BM were detected in 2/20 patients (10%) with CML, both presenting with an accelerated phase of disease (CML‐AP) and both exhibiting the t(9;22) cytogenetic abnormality (Ph chromosome). Cells of TROCI‐BM reacted with the antibody 2D7, but did not express CD117/Kit. These cells were therefore classified as neoplastic basophils leading to the final diagnosis of a “secondary basophilic leukaemia” (fig 1). All other cases of CML were in the chronic phase (CML‐CP) and showed a constant diffuse increase in loosely scattered 2D7 positive cells sometimes forming small clusters, but the criteria for TROCI‐BM were not fulfilled. Altogether, the number of 2D7 positive cells (basophilic granulocytes) was too small to diagnose secondary basophilic leukaemia. Surprisingly, in one case of CML‐CP, spindle shaped tryptase positive cells were found to form a solitary compact infiltrate. Therefore, the distinction from TROCI‐BM was easily achievable and a diagnosis of SM with an associated CML could be established (SM‐AHNMD or SM‐CML, respectively).
Molecular studies
The typical activating point mutation of c‐kit D816V was detected in lesional MC of the bone marrow in seven of the nine cases with indolent SM (ISM) and the case of SM‐AHNMD (SM‐CML). All mutated cases exhibited an aberrant immunophenotype of tryptase positive MC with coexpression of CD25 (fig 1). The remaining two patients with ISM and all the other patients in this study revealed the wild type c‐kit in the relevant hot spot region of exon 17. Of these, the two non‐mutated ISM cases contained MC that were also negative for CD25.
DISCUSSION
Tryptase is a well established immunohistochemical marker in haematopathology and is commonly used for detection of MC in patients with SM.1,3 However, recent data indicate that tryptase may not only be expressed by MC, but also by basophilic granulocytes, especially in neoplastic states, and has even been detected in AML blast cells.18,22 It could be shown clearly that tryptase expression reflects minimal differentiation of blast cells towards basophil and/or MC lineages and that tryptase expression represents a very specific finding, as myeloblasts have been demonstrated by molecular techniques to contain tryptase mRNA.18 The rare finding of compact, tryptase positive, round cell infiltrates in a minority of SM cases prompted us to survey normal/reactive BM and neoplastic BM involved in myeloid malignancies to look for the incidence and cellular composition of TROCI‐BM in detail. To address this issue, we applied a panel of four decisive markers (CD25 (SM related), CD34 (blast cell related), CD117 (MC related), and 2D7 (basophil related)) in 18 patients exhibiting the TROCI‐BM phenomenon. These four markers proved to be sufficient to define the cell type involved in TROCI‐BM.
Most patients with SM exhibit multifocal dense intramedullary infiltrates consisting of spindle shaped tryptase positive MC.1,23,24,25 The minimal diagnostic requirement (one major criterion (multifocal aggregates) plus one minor criterion (spindle shaped MC)) was fulfilled in these cases.6,7 However, in about 10% of all SM cases, the majority of intralesional MC are round, leading to the histopathological phenomenon of TROCI‐BM. The diagnosis of SM in such cases cannot be established on morphological BM findings alone. In these cases, the diagnosis of SM can only be confirmed in a stepwise fashion by immunostaining and application of additional criteria. As the first step, the identity of the MC involved in TROCI‐BM has to be reconfirmed by demonstrating expression of MC related antigens. In this study, CD117 was used as the MC marker, and was found to be consistently expressed by MC in various myeloid neoplasms, a finding that also confirmed previous studies.4,5 To assess or exclude the presence of basophils in the TROCI‐BM, the basophil specific marker 2D7 was used, and was found to be non‐reactive in all SM cases exhibiting CD117 positive TROCI‐BM.
The next step in the diagnostic investigation of suspected SM in patients with TROCI‐BM is the use of an antibody against CD25. It has been described that expression of CD25 on MC is a reliable minor diagnostic criterion of SM, as it can be clearly shown that MC in other myeloid neoplasms or reactive states (MC hyperplasia) do not express CD25.21 Originally, CD25 expression by neoplastic MC was demonstrated by flow cytometry.26 More recently, however, we were able to show that CD25 immunohistochemistry can also be applied to routinely processed BM biopsy specimens, and proved a very reliable immunohistochemical marker for neoplastic MC in SM.21 In the present study, as expected, TROCI‐BM cells coexpressed CD25 and CD117 in a majority of the cases with SM. However, in 2/9 patients, TROCI‐BM cells did not express CD25. The reason for this may be that in these cases, the neoplastic MC expressed only low levels of CD25, which were below the detection level of the applied immunohistochemical staining technique. Unfortunately, we were not able to perform flow cytometry experiments in these cases to clarify whether or not low levels of CD25 were expressed on BM MC. An alternative explanation for the CD25 negative staining result could be that these patients had a recently described, apparently extremely rare subvariant of SM characterised by round MC and the transmembrane c‐kit mutation F522C.27 Accordingly, we were not able to detect the classical c‐kit point mutations D816V even by using microdissected pooled MC in these two patients. Studies are under way to clarify whether or not the F522C mutation is expressed by the CD25 negative BM MC of our two SM patients, who exhibited morphological features of the well differentiated round cell variant of SM.
In contrast to the focal TROCI‐BM infiltrate, diffuse TROCI‐BM is found in MCL as a very rare subvariant of SM, usually with large numbers of circulating MC and a marked increase of MC in BM smears of >20% of all nucleated cells.6,7 A very similar histopathological picture, however, can also be found in patients with MML.28 In contrast to MCL, these patients lack the diagnostic SM criteria and always show an increase in immature atypical MC and partially metachromatic blast cells.15,16,28 In both groups of patients, TROCI‐BM was diffuse and was found to stain positive for anti‐CD117. However, whereas TROCI‐BM cells in MCL were found to be CD34 negative, TROCI‐BM cells in MML were found to be at least partly CD34 positive and lacking CD25. These data confirm the diagnostic value of CD34 and CD25 in the differential diagnosis of leukaemias with involvement of MC lineages (MCL versus MML).
A further differential diagnosis in patients with myeloid leukaemia and diffuse‐type TROCI‐BM is tryptase postitive AML.14,17 Here, TROCI‐BM consists primarily of CD34 positive blast cells, whereas differentiation towards MC or basophils is not easily detectable, in particular diagnostic criteria of SM are not fulfilled. However, it could be recently shown that blast cells in tryptase postitive AML may express MC or basophil related antigens, thereby indicating a minimal differentiation towards MC.18 In the current study, however, TROCI‐BM in tryptase postitive AML were always found to be negative for CD117 and 2D7, indicating a very immature (precommitted) stage of blast cell differentiation.
A major disadvantage in diagnostic haematopathology has been the complete lack of a reliable immunohistochemical marker for basophils. We have recently demonstrated that the 2D7 antigen is specifically expressed by basophils and can therefore be applied as a specific basophil associated immunohistochemical marker in various myeloid neoplasms (unpublished observations). In the present study, we extended these analyses to patients with TROCI‐BM, where the application of the antibody is of crucial importance, at least when these cells are CD117 negative, thus suggesting the presence of basophils. Our staining experiments showed that CD117 positive TROCI‐BM are always 2D7 negative, and 2D7 positive TROCI‐BM are always CD117 negative, which strongly supports the concept that 2D7 is a basophil specific immunohistochemical marker in various myeloid neoplasms.
CML almost always shows an excess of basophils, which is pronounced when the disease is in its accelerated phase and can lead to a picture that is nearly indistinguishable from MML.13,16,29 Therefore, it is of particular importance to define the type of intralesional cells in TROCI‐BM in all patients with accelerated or blast phase CML. In the present study, two patients with CML and TROCI‐BM were identified and further analysed. TROCI‐BM were found to express 2D7 in both cases, but were negative for CD117, enabling us to establish the diagnosis of a secondary basophilic leukaemia. In another patient with chronic phase CML, focal dense infiltrates of tryptase positive cells were found to stain positive for CD117, but did not react with 2D7. As the tryptase positive cells were spindle shaped, TROCI‐BM could be easily excluded and a final diagnosis of SM with associated CML (SM‐AHNMD) established.
In reactive states or some low grade non‐Hodgkin's lymphomas, the numbers of MC may be very high.30 However, MC hyperplasia is not accompanied by the formation of TROCI‐BM, with the extremely rare exception of a case of stem cell induced MC hyperplasia.31 Therefore, TROCI‐BM can be regarded as almost indicative of the presence of a myeloid neoplasm. The application of additional markers is crucial for proper haematopathological investigation.
Since we identified TROCI‐BM, all new cases of myeloid neoplasms (including MDS, MPS, MDS/MPS, and AML) are now routinely stained with anti‐tryptase to obtain more information about the exact frequency and impact of this finding in the form of a prospective study which is planned for a span of about 5 years.
To summarise, the finding of TROCI‐BM is extremely rare but strongly suggestive of a myeloid neoplasm including six rare differential diagnoses. We found that application of a limited panel of antibodies directed against various MC and basophil related antigens such as CD25, CD34, CD117, and 2D7 is sufficient to define the cell type involved in TROCI‐BM with certainty.
TAKE HOME MESSAGES
Focal compact or diffuse infiltrates consisting of tryptase positive round cells (“TROCI‐BM”) indicate the presence of a myeloid neoplasm, but represent a very rare histomorphological phenomenon occurring only in a small minority of patients with SM, AML, or CML.
To clearly define the cell types involved in TROCI‐BM, it is crucial to apply a panel of antibodies against mast cell and basophil related markers, including CD25, CD117, and the 2D7 antigen.
In patients with multifocal compact TROCI‐BM consisting of MC (CD117+, 2D7−), expression of CD25 confirms their neoplastic immunophenotype and allows the diagnosis of SM. In very rare cases of SM, however, TROCI‐BM MC may be CD25 negative.
In patients with diffuse TROCI‐BM consisting of MC (CD117+, 2D7−), the final diagnosis is MCL or MML, depending on expression of CD25 and presence or absence of other SM criteria.
In patients with diffuse TROCI‐BM consisting of CD34 positive myeloblasts, the final diagnosis is tryptase postitive AML or MML, depending on the percentage of immature MC (including metachromatic blast cells).
Expression of 2D7 in TROCI‐BM cells is indicative of a neoplastic population of basophilic granulocytes, and was seen only in a small minority of patients with accelerated phase CML (“secondary basophilic leukaemia”).
The activating point mutation D816V was detected only in patients with common type SM (ISM and SM‐AHNMD) exhibiting MC with an aberrant immunophenotype and coexpression of CD25 but could not be demonstrated in any of the other haematological malignancies associated with TROCI‐BM.
ACKNOWLEDGEMENT
This study was upported by the Fond Zur Förderung der Wissenschaftlichen Forschung in Österreich and the Fortüne Förderprogramm der Universität Tübingen (grant no. F1461141). H Agis is a recipient of an FWF Charlotte Bühler grant (no. H‐185‐B04).
Abbreviations
AML - acute myeloid leukaemia
CML - chronic myeloid leukaemia
CMLba - chronic myeloid leukaemia with excess of basophils
ISM - indolent systemic mastocytosis
MCL - mast cell leukaemias
MML - myelomastocytic leukaemia
SM - systemic mastocytosis
TROCI‐BM - tryptase‐positive compact round cell infiltrate of the bone marrow
References
- 1.Horny H‐P, Sillaber C, Menke D.et al Diagnostic value of immunostaining for tryptase in patients with mastocytosis. Am J Surg Pathol 1998221132–1140. [DOI] [PubMed] [Google Scholar]
- 2.Li C Y. Diagnosis of mastocytosis: value of cytochemistry and immunohistochemistry. Leuk Res 200125537–541. [DOI] [PubMed] [Google Scholar]
- 3.Li W V, Kapadia S B, Sonmez‐Alpan E, Swerdlow S H. Immunohistochemical characterization of mast cell disease in paraffin sections using tryptase, CD68, myeloperoxidase, lysozyme, and CD20 antibodies. Mod Pathol 19969982–988. [PubMed] [Google Scholar]
- 4.Horny H‐P, Valent P. Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res 200125543–551. [DOI] [PubMed] [Google Scholar]
- 5.Horny H‐P, Valent P. Histopathological and immunohistochemical aspects of mastocytosis. Int Arch Allergy Immunol 2002127115–117. [DOI] [PubMed] [Google Scholar]
- 6.Valent P, Horny H‐P, Escribano L.et al Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 200125603–625. [DOI] [PubMed] [Google Scholar]
- 7.Valent P, Horny H‐P, Li C Y.et al Mastocytosis (Mast cell disease). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of haematopoietic and lymphoid tissues 2001291–302.
- 8.Fukuda T, Kamishima T, Tsuura Y.et al Expression of the c‐kit gene product in normal and neoplastic mast cells but not in neoplastic basophil/mast cell precursors from chronic myelogenous leukaemia. J Pathol 1995177139–146. [DOI] [PubMed] [Google Scholar]
- 9.Jordan J H, Walchshofer S, Jurecka W.et al Immunohistochemical properties of bone marrow mast cells in systemic mastocytosis: evidence for expression of CD2, CD117/Kit, and bcl‐x(L). Hum Pathol 200132545–552. [DOI] [PubMed] [Google Scholar]
- 10.Kepley C L, Craig S S, Schwartz L B. Identification and partial characterization of a unique marker for human basophils. J Immunol 19951546548–6555. [PubMed] [Google Scholar]
- 11.Bennett J M, Catovsky D, Daniel M T.et al Proposals for the classification of the acute leukaemias. French‐American‐British (FAB) co‐operative group. Br J Haematol 197633451–458. [DOI] [PubMed] [Google Scholar]
- 12.Bennett J M, Catovsky D, Daniel M T.et al Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French‐American‐British Cooperative Group. Ann Intern Med 1985103620–625. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe E S, Harris N L, Stein H, Vardiman J W. eds. World Health Organization (WHO) classification of tumours. Pathology and genetics. Tumours of haematopoietic and lymphoid tissues . 2001;1291–302.
- 14.Sperr W R, Horny H‐P, Lechner K.et al Clinical and biologic diversity of leukemias occurring in patients with mastocytosis. Leuk Lymphoma 200037473–486. [DOI] [PubMed] [Google Scholar]
- 15.Valent P, Samorapoompichit P, Sperr W R.et al Myelomastocytic leukemia: myeloid neoplasm characterized by partial differentiation of mast cell‐lineage cells. Hematol J 2002390–94. [DOI] [PubMed] [Google Scholar]
- 16.Valent P, Spanblöchl E, Bankl H C.et al Kit ligand/mast cell growth factor‐independent differentiation of mast cells in myelodysplasia and chronic myeloid leukemic blast crisis. Blood 1994844322–4332. [PubMed] [Google Scholar]
- 17.Sperr W R, Hauswirth A W, Valent P. Tryptase a novel biochemical marker of acute myeloid leukemia. Leuk Lymphoma 2002432257–2261. [DOI] [PubMed] [Google Scholar]
- 18.Sperr W R, Jordan J H, Baghestanian M.et al Expression of mast cell tryptase by myeloblasts in a group of patients with acute myeloid leukemia. Blood 2001982200–2209. [DOI] [PubMed] [Google Scholar]
- 19.Hsu S M, Raine L, Fanger H. Use of avidin‐biotin‐peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 198129577–580. [DOI] [PubMed] [Google Scholar]
- 20.Sotlar K, Fridrich C, Mall A.et al Detection of c‐kit point mutation Asp‐816→Val in microdissected pooled single mast cells and leukemic cells in a patient with systemic mastocytosis and concomitant chronic myelomonocytic leukemia. Leuk Res 200226979–984. [DOI] [PubMed] [Google Scholar]
- 21.Sotlar K, Horny H ‐ P, Simonitsch I.et al CD25 indicates the neoplastic phenotype of mast cells: a novel immunohistochemical marker for the diagnosis of systemic mastocytosis (SM) in routinely processed bone marrow biopsy specimens. Am J Surg Pathol 2004281319–1325. [DOI] [PubMed] [Google Scholar]
- 22.Samorapoompichit P, Kiener H P, Schernthaner G H.et al Detection of tryptase in cytoplasmic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood 2001982580–2583. [DOI] [PubMed] [Google Scholar]
- 23.Horny H ‐ P, Parwaresch M R, Lennert K. Bone marrow findings in systemic mastocytosis. Hum Pathol 198516808–814. [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe D D. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol 1991962–4S. [PubMed] [Google Scholar]
- 25.Parwaresch M R, Horny H P, Lennert K. Tissue mast cells in health and disease. Pathol Res Pract 1985179439–461. [DOI] [PubMed] [Google Scholar]
- 26.Escribano L, Orfao A, Diaz‐Agustin B.et al Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood 1998912731–2736. [PubMed] [Google Scholar]
- 27.Akin C, Fumo G, Yavuz A S.et al A novel form of mastocytosis associated with a transmembrane c‐kit mutation and response to imatinib. Blood 20041033222–3225. [DOI] [PubMed] [Google Scholar]
- 28.Valent P, Sperr W R, Samorapoompichit P.et al Myelomastocytic overlap syndromes: biology, criteria, and relationship to mastocytosis. Leuk Res 200125595–602. [DOI] [PubMed] [Google Scholar]
- 29.Agis H, Beil W J, Bankl H C.et al Mast cell‐lineage versus basophil lineage involvement in myeloproliferative and myelodysplastic syndromes: diagnostic role of cell‐immunophenotyping. Leuk Lymphoma 199622187–204. [DOI] [PubMed] [Google Scholar]
- 30.Prokocimer M, Polliack A. Increased bone marrow mast cells in preleukemic syndromes, acute leukemia, and lymphoproliferative disorders. Am J Clin Pathol 19817534–38. [DOI] [PubMed] [Google Scholar]
- 31.Jordan J H, Schernthaner G H, Fritsche‐Polanz R.et al Stem cell factor‐induced bone marrow mast cell hyperplasia mimicking systemic mastocytosis (SM): histopathologic and morphologic evaluation with special reference to recently established SM‐criteria. Leuk Lymphoma 200243575–582. [DOI] [PubMed] [Google Scholar]