Abstract
Objective
To investigate infiltrating cells in the liver of children with type 1 autoimmune hepatitis (AH‐1).
Methods
liver biopsies from 24 untreated AH‐1 patients (14 children, 10 adults), five patients with hepatitis C virus related chronic hepatitis (HCV), and 10 control liver specimens (CL) were processed for immunohistochemical cell characterisation.
Results
Two different cell distribution patterns were detected in the liver of patients with AH‐1: (1) CD4+ and CD20+ cells were found in the central areas of the portal tracts (portal distribution); (2) CD8+ cells were observed at the periphery of the portal space (periportal distribution). Some cell subsets, like CD56, CD57, Fas‐L, and Bak, showed a non‐defined distribution pattern. The presence of two well defined patterns of cell distribution was not observed in HCV and CL (CD4+, CD20+, and CD8+ cells were uniformly distributed in the portal space). In AH‐1 and CL, the NK markers CD56 and CD57 were found scattered throughout the liver parenchyma. However, in HCV biopsies, CD56+ cells were also clearly increased in both the portal and the periportal areas. Biopsies of AH‐1 and HCV patients showed a uniform distribution of Fas‐L and Bak in the portal and periportal areas, with Bak staining also detected in the hepatic parenchyma.
Conclusions
Despite clinical and genetic differences, there was a similar distribution of liver infiltrating mononuclear cells in children and adults with AH‐1. These results raise the possibility of reclassifying cryptogenic chronic hepatitis by immunohistochemical analysis of infiltrating liver cells.
Keywords: type 1 autoimmune hepatitis, immunohistochemistry, mononuclear cell infiltrate, children
Autoimmune liver disorders are progressive inflammatory diseases that usually respond to immunosuppressive treatment.1,2,3 Type 1 autoimmune hepatitis in paediatric patients (PAH)4 is the major cause of chronic liver disease in Argentinian children.5 This entity is serologically characterised by the presence of specific autoantibodies, increased levels of immunoglobulin G, and a mononuclear cell infiltration, which is believed to play a role in the maintenance of liver damage. The characterisation of this cellular infiltrate has been extensively investigated in several liver diseases. However, is limited the information on Type 1 autoimmune hepatitis (AH‐1).6,7,8,9,10 These studies—which showed the presence of CD4 positive T cells in the portal spaces and an aberrant expression of HLA class II antigens in the liver of adult patients—suggested the involvement of T helper cells in the pathogenesis of AH‐1.11,12 Some studies have also suggested an antibody mediated pathogenesis, but the putative hepatic autoantigen remains to be characterised.
Several reports from our own laboratory have identified genetic and clinical differences between PAH and adult patients with AH‐1 (AAH) which indicate that they may represent different clinical entities.13,14,15,16 The comparison between PAH and AAH showed that the HLA‐DRB1*1301‐DQB1*0603 haplotype is strongly associated with PAH, while HLA‐DR3 and HLA‐DR4 represent the HLA susceptibility genes associated with AAH.14,17 In addition, PAH represent a severe disease with a more severe outcome, despite the use of higher doses of immunosuppressive agents.13
This study was designed to characterise the composition of the mononuclear cell subpopulations infiltrating the liver in PAH. We are aware that although interactions between the different infiltrating cellular subsets might indicate a putative pathogenic mechanism, its functional relevance is difficult to extrapolate simply by studying the immunophenotypes.
Methods
Liver biopsies were obtained at diagnosis in 14 PAH and 10 AAH cases (none on immunosuppressive therapy) and from five children with HCV who had been off antiviral therapy for at least three months. AH‐1 patients fulfilled clinical, laboratory, histological, and immunological criteria for diagnosis.18 Anti‐HCV antibodies were confirmed by enzyme linked immunosorbent assay (ELISA) or immunoblot tests. AH‐1 samples included are representative and were randomly chosen from our previous study, which contains the detailed clinical data from 122 paediatric and 84 adult patients.13 Control liver (CL) sections were obtained from cadaveric donors (median age 16 years, range 7 to 30) by agreement with the Central Unique National Institute Coordinator of Ablation and Implant. All had normal transaminases values at death, as previously reported.19,20 The ethics review board at each author's institution approved this study. Informed consent was obtained from patients' parents or relatives.
Tissue specimens fixed in formalin and embedded in paraffin were processed for standard histological examination. We graded hepatic necroinflammatory activity, and staged fibrosis and architectural distortion by a numerical score.21,22
Immunohistochemistry
On average, 12 serial sections of each tissue block were stained using an immunoperoxidase system (ABC kit Elite, Vectastain® Universal); primary monoclonal antibodies were anti‐CD45 (IgG1), anti‐CD20 (IgG2a) (Dako, Carpinteria, California, USA), anti‐CD3 (IgG2a), anti‐CD4 (IgG1), anti‐CD8 (IgG2b), anti‐Fas‐L (IgM), anti‐CD57 (IgM) (Novocastra Laboratories, Newcastle Upon Tyne, UK), anti‐CD56 (IgG1) (Santa Cruz Biotechnology, California, USA), and polyclonal IgG anti‐Bak. Negative controls omitted primary antibodies. Sections were deparaffinised and rehydrated, endogenous peroxidase activity blocked (methanol‐3% H2O2), and preincubated with horse serum (Vector Laboratories, Burlingame, California, USA). Some antigens required previous treatment in a microwave oven (CD20, CD3, Fas‐L, and Bak: 10 mM sodium citrate buffer, pH 6.0; CD4 and CD8: 1 mM methylene diamine tetra‐acetate (EDTA), pH 8.0.23 CD45, CD3, and Fas‐L were diluted 1:100; CD4 and CD8, 1:20; CD56, 1:25; CD57, 1:50; and Bak, 1:400 in phosphate buffered saline and incubated overnight at 4°C. For CD4 and CD8 detection, biotinylated antibody (Vectastain® Universal Elite®, Vector Laboratories), alkaline phosphatase conjugated ABC (Vectastain® ABC‐AP kit) and alkaline phosphatase substrate kit (BioRad, Hercules, California, USA) were used. For the remainder antigens, biotinylated antibody (Vectastain® Universal Elite®, Vector Laboratories), horseradish peroxidase conjugated ABC (Vectastain® ABC kit, Vector Laboratories), and 3,3′diaminobenzidine substrate kit (Novocastra Laboratories) were used. Sections were counterstained with 10% Harris's haematoxylin.
Statistical analysis
Stained cells were observed at high magnification, five fields on average for each area, and assessed on a five point scale (0, 0–5%; 1, 5–20%; 2, 20–50%; 3, 50–80%; and 4, 80–100%); 2×2 contingency tables were constructed and χ2 or Fischer's exact test were used as appropriate. The non‐parametric Mann–Whitney U test was also used as indicated.
Results
Table 1 summarises the laboratory features at presentation in all the patients in the study. Gamma globulins differed significantly between PAH and AAH. Though the majority of patients had hypergammaglobulinaemia, children showed significantly higher levels than adults. The laboratory findings were in keeping with our previously published results.13 Anti‐smooth muscle antibodies (SMA) were more frequent in PAH than AAH (100% v 60%, p = 0.019). The combined presence of antinuclear antibodies (ANA) and SMA was observed in PAH and AAH (36% v 50%, NS). ANA were more common in AAH than in PAH (80% v 36%, p = 0.047). An acute viral hepatitis‐like presentation predominated in PAH over AAH (78% v 30%, p = 0.03). As previously reported, HLA‐DRB1*1301 predominated in PAH and HLA‐DRB1*0405 in AAH (78% and 70%). Table 2 shows the histological features of all patients on entering the study.
Table 1 Laboratory features at presentation of patients with type 1 autoimmune hepatitis and hepatitis C.
| PAH | AAH | p Value† | HCV | |
|---|---|---|---|---|
| No of patients | 14 | 10 | 5 | |
| Male/female (n) | 4/10 | 2/8 | 3/2 | |
| Age at diagnosis (years)* | 7 (2 to 12) | 48 (42 to 52) | 6 (2 to 13) | |
| Total protein (g/dl) (nv 6–8)* | 8.31 (6.7 to 10.4) | 6.4 (4.9 to 9.7) | NS | 7.7 (6.9 to 8.2) |
| AST (IU/l) (nv <50)* | 568.8 (71 to 1204) | 507.5 (116 to 883) | NS | 52.1(19 to 84) |
| ALT/GTP (IU/l) (nv <50)* | 537.7 (69 to 990) | 703.6 (70 to 1091) | NS | 70 (24 to 132) |
| Alkaline phosphatase (IU/l) (nv <350)* | 688.36 (530 to 1078) | 685.2 (333 to 740) | NS | 684 (307 to 1169) |
| γ‐Glutamyl transferase (IU/l) (nv <50)* | 144 (48 to 249) | 196(82 to 279) | NS | 42 (6 to 75) |
| γ‐Globulins (g%) (nv <1.8) | 4.5 (2.41 to 8.3) | 1.8 (1.3 to 2.08) | 0.007 | 1.5 (1.1 to 1.74) |
| Prothrombin time (%) (nv = 100)* | 74 (50 to 92) | 86(68 to 100) | NS | 100 |
| PTT (s) | 40 (31 to 56) | 42.5(33 to 49) | NS | 33(32 to 34) |
| Total bilirubin (μmol/l) (nv <24)* | 52.3 (10.3 to 177.8) | 51.0 (13.7 to 152.2) | NS | 12.0 (10.3 to 17.1) |
*Values expressed as median (range).
†PAH v AAH, two tail non‐parametric Mann‐Whitney test.
AAH, adult autoimmune hepatitis type 1; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, patients with hepatitis C virus‐related chronic hepatitis; nv, normal value; PAH, paediatric autoimmune hepatitis type 1; PTT, partial thromboplastin time.
Table 2 Description of the chronic hepatitis in patients included in this study.
| Patient | Description | HAI* | Stage† | Patient | Description | HAI* | Stage† | Patient | Description | HAI* | Stage† |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH 1 | Severe | 15 | 4 | AAH 1 | Moderate | 9 | 2 | HCV 1 | Mild | 5 | 1 |
| PAH 2 | Severe | 14 | 4 | AAH 2 | Moderate | 9 | 5 | HCV 2 | Mild | 5 | 2 |
| PAH 3 | Moderate | 11 | 5 | AAH 3 | Severe | 14 | 4 | HCV 3 | Mild | 7 | 2 |
| PAH 4 | Moderate | 10 | 3 | AAH 4 | Severe | 13 | 4 | HCV 4 | Moderate | 7 | 3 |
| PAH 5 | Moderate | 11 | 3 | AAH 5 | Severe | 13 | 4 | HCV 5 | Mild | 5 | 1 |
| PAH 6 | Severe | 14 | 4 | AAH 6 | Severe | 13 | 6 | ||||
| PAH 7 | Severe | 13 | 4 | AAH 7 | Moderate | 9 | 2 | ||||
| PAH 8 | Severe | 14 | 3 | AAH 8 | Severe | 14 | 3 | ||||
| PAH 9 | Severe | 14 | 4 | AAH 9 | Severe | 15 | 4 | ||||
| PAH 10 | Severe | 16 | 5 | AAH10 | Moderate | 7 | 1 | ||||
| PAH 11 | Severe | 14 | 5 | ||||||||
| PAH 12 | Severe | 14 | 4 | ||||||||
| PAH 13 | Mild | 8 | 4 | ||||||||
| PAH 14 | Moderate | 10 | 4 |
*HAI, histological activity index (highest score = 18): severe, HAI >12; moderate, HAI >9 but <12; mild, HAI <9.
†Stages 1–5: fibrosis; stage 6: definitive cirrhosis.
AAH, adult autoimmune hepatitis type 1; HCV, patients with hepatitis C virus related chronic hepatitis; PAH, paediatric autoimmune hepatitis type 1.
Analysis of mononuclear cell subpopulations
Table 3 gives the scores most often found in PAH, AAH, HCV, and control liver. Percentages of stained cells were scored (see Methods) and used to describe the pattern of distribution of cellular subpopulations. Two different cellular patterns were detected in AH‐1: CD4+ and CD20+ cells in central areas of the portal tracts (portal distribution) and CD8+ cells at the periphery of the portal tracts (periportal distribution). Some cellular subsets (CD56, CD57, Fas‐L, Bak) showed an undefined distribution pattern. The two well defined distribution patterns were not observed in HCV and control liver (CD4+, CD20+, and CD8+ cells were uniformly distributed in the portal tracts). In AH‐1 and control liver, the NK (natural killer cell) markers CD56 and CD57 were found scattered through the liver parenchyma. However, in HCV biopsies, CD56+ cells were clearly increased in the portal and periportal areas. Biopsies from AH‐1 and HCV individuals showed a uniform distribution of Fas‐L and Bak in the portal spaces, with Bak staining also detected in the hepatic parenchyma.
Table 3 Distribution of cellular subpopulations in three areas of the liver using different antibodies.
| No | CD20 | CD4 | CD8 | CD56 | CD57 | FasL | Bak | |
|---|---|---|---|---|---|---|---|---|
| A. Central areas of portal tracts | ||||||||
| PAH | 14 | 2 *(93%) | 3(79%) | 1 (64%) | 1 (100%) | 1 (100%) | 2 (79%) | 1 (57%) |
| 2 (36%) | 2 (43%) | |||||||
| AAH | 10 | 2 (100%) | 3 (80%) | 1 (80%) | 1 (100%) | 1 (100%) | 2 (90%) | 1 (60%) |
| 2 (20%) | 2 (40%) | |||||||
| HCV | 5 | 2 (80%) | 2 (100%) | 2 (100%) | 2 (60%) | 1 (80%) | 2 (80%) | 2 (80%) |
| 3 (40%) | ||||||||
| CL | 10 | 2 (100%) | 2 (80%) | 2 (70%) | 1 (90%) | 0 (90%) | 0 (60%) | 1 (70%) |
| 2 (50%) | 1 (20%) | |||||||
| B. Periportal areas | ||||||||
| PAH | 14 | 1* (86%) | 1 (79%) | 3 (100%) | 1 (100%) | 1 (100%) | 2 (79%) | 1 (57%) |
| 2 (43%) | ||||||||
| AAH | 10 | 1 (100%) | 1 (80%) | 3 (100%) | 1 (100%) | 1 (100%) | 2 (90%) | 1 (60%) |
| 2 (40%) | ||||||||
| HCV | 5 | 2 (80%) | 2 (100%) | 2 (100%) | 2 (60%) | 1 (80%) | 2 (80%) | 2 (80%) |
| 3 (40%) | ||||||||
| CL | 10 | 1 (100%) | 2 (80%) | 2 (70%) | 1 (90%) | 0 (90%) | 1 (80%) | 1 (70%) |
| C. Lobular areas | ||||||||
| PAH | 14 | 1*(86%) | 1 (100%) | 2 (64%) | 1 (100%) | 1 (100%) | 1 (86%) | 2 (93%) |
| 3 (36%) | ||||||||
| AAH | 10 | 1 (90%) | 1 (100%) | 2 (50%) | 1 (100%) | 1 (100%) | 1 (80%) | 2 (90%) |
| 3 (50%) | ||||||||
| HCV | 5 | 1 (80%) | 2 (100%) | 1 (80%) | 1 (100%) | 1 (80%) | 2 (80%) | 2 (80%) |
| CL | 10 | 1 (100%) | 2 (100%) | 0 (100%) | 1 (100%) | 0 (90%) | 0 (60%) | 1 (70%) |
| 1 (20%) | ||||||||
Percentages of stained cells were independently analysed in different areas of the liver. *Scores found in at least 70% of biopsies analysed. The percentages of biopsies with each score (*) are indicated between brackets for each marker. As described in Methods the score was assessed on a five point scale: 0, 0–5%; 1, 5–20%; 2, 20–50%; 3, 50–80%; 4, 80–100%.
No, total number of patients.
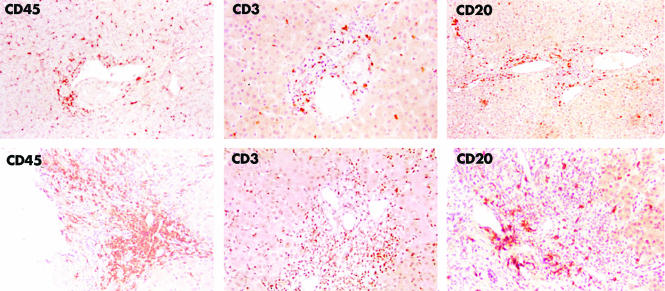
As expected, the number of CD45+ cells in each biopsy agreed with the histological activity index (table 2). Cadaveric CL showed a mononuclear cell infiltrate, probably ascribable to inflammatory events induced around the time of brain death.24 However, the cellular composition in control liver clearly differed from pathological samples (table 3, figs 1–3). Figure 1 shows a representative PAH biopsy and a control liver.
Figure 1 Upper panel: representative immunohistochemical staining for CD45, CD3, and CD20 expression in a control liver specimen. Positive staining is shown in all areas of the liver. Lower panel: representative immunohistochemical staining for the same markers in biopsies from paediatric patients: CD45 (PAH 1); CD3 and CD20 (PAH 9). CD45 and CD3 localise to portal tracts and liver parenchyma, positive CD20 staining shows a portal pattern of distribution.
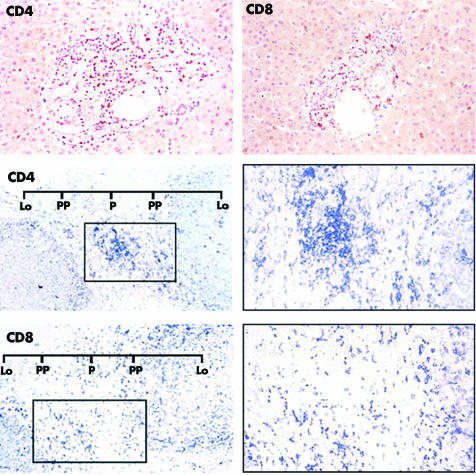
Figure 2 Upper panel: representative immunohistochemical staining for CD4 and CD8 expression in a control liver specimen. Positive staining is uniformly distributed in the portal tracts. Middle and lower panels: representative immunohistochemical staining for the same markers in biopsies from a paediatric patient (PAH4). Middle panel: CD4 positive staining shows a portal pattern of distribution. Original magnification ×200, left; ×400, right. Lower panel: CD8 positive staining shows a periportal pattern of distribution. Original magnification ×200, left; ×400, right. Lo, lobule; P, central area of the portal tract; PP, periportal area.
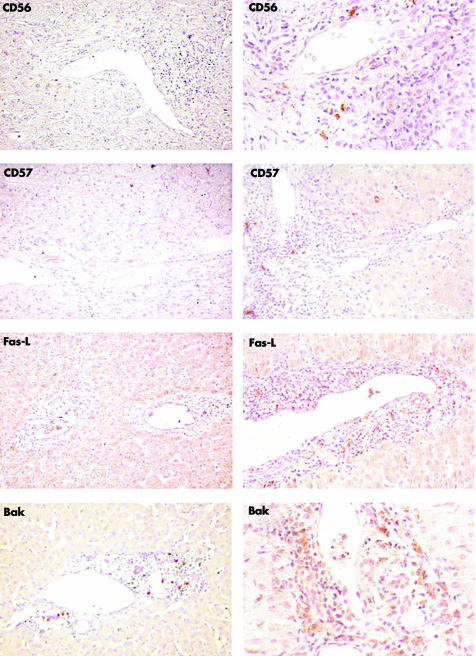
Figure 3 Left panel: representative immunohistochemical staining for CD56, CD57, Fas‐L, and Bak expression in a control liver specimen. Scattered CD56, CD57, and Fas‐L positive stained cells are observed in the parenchyma. Bak positively stained cells are clearly observed in portal and periportal areas. Kupffer cells stain positive in the parenchyma. Right panel: representative immunohistochemical staining for the same markers, in biopsies from paediatric patients (CD56 and CD57, PAH9; Fas‐L and Bak, PAH5). Scattered CD56 and CD57 positive cells are observed in the portal tracts. Fas‐L and Bak stain positive in the three areas of the liver.
In AH‐1, no correlation between histological progression of the chronic hepatitis (severe or moderate biopsies) and the composition of the cellular infiltrate was found. In spite of genetic and clinical differences, cellular distribution patterns did not differ between PAH and AAH. Statistical evaluation of CD20, CD4, and CD8 stained cell distributions is given in table 4.
Table 4 Statistical analysis of the distribution of cellular subpopulations.
| Frequency * | Significance† | |
|---|---|---|
| A. Incidence of biopsies with a score = 2 for CD20 stained cells | ||
| PAH | ||
| Portal tracts | 13/14 (92.85%) | p<0.0001‡;§ |
| Periportal area | 0/14 (0%) | |
| Lobular area | 0/14 (0%) | |
| AAH | ||
| Portal tracts | 10/10 (100%) | p<0.0001‡;§ |
| Periportal area | 0/10 (0%) | |
| Lobular area | 0/10 (0%) | |
| B. Incidence of biopsies with a score = 3 for CD4 stained cells | ||
| PAH | ||
| Portal tracts | 11/14 (78.57%) | p<0.0001‡;§ |
| Periportal area | 0/14 (0%) | |
| Lobular area | 0/14 (0%) | |
| AAH | ||
| Portal tracts | 8/10 (80%) | p<0.02301‡;§ |
| Periportal area | 2/10 (20%) | |
| Lobular area | 2/10 (20%) | |
| C. Incidence of biopsies with a score = 3 for CD8 stained cells | ||
| PAH | ||
| Portal tracts | 0/14 (0%) | |
| Periportal area | 14/14 (100%) | p<0.0001‡; p = 0.0006§ |
| Lobular area | 5/14 (5%) | |
| AAH | ||
| Portal tracts | 0/10 (0%) | |
| Periportal area | 10/10 (100%) | p<0. 0001‡; p = 0.0325§ |
| Lobular area | 5/10 (50%) | |
A: *Number of cases with score = 2/number of total cases. The percentages obtained for each score (*) are in brackets.
†Two tailed Fischer's exact test; †v periportal area, ††v lobular area.
B: *Number of cases with score = 3/number of total cases. The percentages obtained for each score (*) are in brackets.
†Two tailed Fischer's exact test; †v periportal area, ††v lobular area.
C: *Number of cases with score = 3/number of total cases. The percentages obtained for each score (*) are in brackets.
†Two tailed Fischer's exact test; ‡v portal area, §v lobular area.
Portal tracts
The CD3 positive subpopulation predominated in the portal tracts (fig 1, lower panel). CD4 and CD20 stained cells had a portal distribution, decreasing in number towards periportal and lobular areas. There was a significantly higher (p<0.0001) incidence of a score of 2 (CD20) or 3 (CD4) in the portal tracts v periportal and lobular areas from PAH and AAH (table 4, A and B). In contrast, CD4 and CD20 positive cells were uniformly distributed in HCV biopsies and control liver (fig 2, upper panel). CD4 stained (fig 2, middle panel) and CD20 stained (fig 1, lower panel) cells were equally represented in AH‐1. These cells largely predominated over the CD8 positive subpopulation (fig 2, lower panel), the sparse number of CD56 and CD57 stained cells (fig 3, right panel), and the Vα24 positive cells previously reported by us.25
Periportal and lobular areas
The CD8 positive subpopulation (fig 2, lower panel) had a periportal distribution in AH‐1 with predominance over CD4 (fig 2, middle panel), CD20 (fig 1, lower panel), CD56 and CD57 (fig 3, right panel), and Vα24.25 Accordingly, the incidence of a score of 3 for CD8 was significantly higher (p<0.0001) in periportal than in portal tracts (table 4C). Of note, CD8 positive cells were also predominant in lobular areas (table 3C). Furthermore, a score of 3 in 36% (PAH) and 50% (AAH) appeared as a specific finding, as a predominant score of 1 was observed in HCV biopsies (table 3C). In control liver, CD8 stained cells were uniformly distributed in the portal and periportal areas but rarely identified in the parenchyma (fig 2, upper panel). As in control liver (fig 3, left panel), a small number of CD56 or CD57 stained cells was homogeneously distributed in PAH (fig 3, right panel). In HCV biopsies, however, CD56 (but not CD57) had higher portal/periportal scores (table 3, A and B).
We also investigated the expression of two functional markers, Fas‐L and Bak. Fas‐L, which identifies a heterogeneous subset of activated cells (LT, NKT, LB), and Bak, which identifies a heterogeneous subpopulation of cells committed to apoptosis, were uniformly distributed through the portal and periportal areas in AH‐1, HCV, and control liver (table 3, fig 3). In all samples, Kupffer cells showed expression of Bak.
Discussion
This is the first immunohistochemical characterisation of the inflammatory infiltrate in biopsies from PAH. In keeping with results obtained in AAH by other investigators,7 our statistical assessment confirms the actual distribution of cellular subpopulations. Strikingly, no differences were found between PAH and AAH. Clinical presentation, gamma globulin levels, autoantibody patterns, and the genetic association of randomly selected PAH and AAH confirm their previously reported identification as different clinical entities.13 However, we were unable to show different patterns of liver infiltrating cells underlying the differences between the two entities.
The portal distribution of CD4 and CD20 positive subpopulations permits their close contact, as happens in lymphoid follicles of lymph nodes or intraportal lymphoid nodules in HCV.8 Following this contact, after recognition of self antigenic peptides by locally activated CD4+ cells, cytokines might be released and liver damage orchestrated by directing clonal proliferation of plasma cells. In fact, circulating autoantibodies and the severity of hepatic lesions are positively correlated in AH‐126 and autoantibodies might also recruit killer cells.27 The moderate number of CD8+ cells present in the portal tracts could ensure interaction with LT helpers and activation of memory effectors. Of note, CD45Ro stained cells are present in AH‐1 infiltrates (not shown). In contrast to HCV biopsies, the lobular (table 3C) and periportal (table 3B) prevalence of CD8+ cells suggested their role as mediators of hepatocyte damage during AH‐I.
Apoptosis by the Fas lytic pathway is the most common death pathway during acute and chronic liver diseases. Accordingly, we found a similar distribution of Fas‐L expressing cells in AH‐1 and HCV biopsies.28,29 Bak staining in Kupffer cells might be derived from apoptotic bodies located inside these cells or from their own apoptotic activity.30,31 Bak staining in portal areas might originate from apoptotic T lymphocytes.32,33
Thus we provide the first description of a specific mononuclear infiltrate in AH‐1 biopsies that is responsible for the liver injury. We are aware that although interactions between infiltrating cellular subsets might indicate a pathogenic mechanism, its functional relevance is difficult to extrapolate by simply studying their immunophenotypes. For this reason the events behind the morphological findings remain speculative. Thus in HCV patients, for example, we found a predominance of CD56 positive cells in the portal and periportal areas. However, in HCV glycoprotein E2 inhibits NK activity by CD81 triggering on the cell surface, possibly contributing to the lack of virus clearance and to the establishment of chronic infection.34,35
In summary, though children and adults with autoimmune hepatitis type 1 have well defined clinical and genetic differences, we showed a remarkably similar distribution of infiltrating mononuclear cells in the liver. This raises the possibility of re‐classifying cryptogenic chronic hepatitis by immunohistochemical analysis of infiltrating liver cells.
Take home messages
While children and adults with autoimmune hepatitis type 1 have well defined clinical and genetic differences, there is a remarkably similar distribution of infiltrating mononuclear cells in the liver.
The presence of definite patterns of cell distribution in the liver raises the possibility of re‐classifying cryptogenic chronic hepatitis by immunohistochemical analysis of infiltrating liver cells.
Acknowledgements
This work was supported by grants from the Buenos Aires University (M018), CONICET (PIP 2116), and Grant “Ramon Carrillo/Arturo Oñativia” of the Public Health Ministry, Argentina. We thank Silvia García for the artwork.
Abbreviations
AAH - adult autoimmune hepatitis type 1
AH‐1 - type 1 autoimmune hepatitis
ANA - antinuclear antibodies
CL - control liver specimen
HCV - hepatitis C virus related chronic hepatitis
PAH - paediatric autoimmune hepatitis type 1
SMA - smooth muscle antibodies
References
- 1.Mackay I R. Immunological aspects of chronic active hepatitis. Hepatology 19833724–728. [DOI] [PubMed] [Google Scholar]
- 2.Czaja A J. Natural history, clinical features, and treatment of autoimmune hepatitis. Seminars in Liver Disease 198441–12. [DOI] [PubMed] [Google Scholar]
- 3.Johnson P J, McFarlane I G. Meeting report: International Autoimmune Hepatitis Group. Hepatology 199318998–1005. [DOI] [PubMed] [Google Scholar]
- 4.Mieli‐Vergani G, Vergani D. Immunological liver diseases in children. Semin Liver Dis 199818271–279. [DOI] [PubMed] [Google Scholar]
- 5.Cuarterolo M, García de Davila M T, Roig A H.et al Hepatitis autoinmune, 8 años de experiencia. Med Infant 199511237–241. [Google Scholar]
- 6.Di Sapio M, Focareta R, Caporaso N.et al Phenotypic characterization of mononuclear cell infiltrate in chronic active hepatitis (CAH) of different etiology. Ric Clin Lab 198717229–234. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto E, Lindor K D, Homburger H A.et al Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Clin Proc Child Hosp Natl Med Cent 1993681049–1055. [DOI] [PubMed] [Google Scholar]
- 8.Mosnier J F, Degott C, Marcellin P.et al The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology 199317366–371. [PubMed] [Google Scholar]
- 9.Eggink H F, Houthoff H J, Huitema S.et al Cellular and humoral immune reactions in chronic active liver disease. I. Lymphocyte subsets in liver biopsies of patients with untreated idiopathic autoimmune hepatitis, chronic active hepatitis B and primary biliary cirrhosis. Clin Exp Immunol 19825017–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Bach N, Thung S N, Schnaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology 199215572–577. [DOI] [PubMed] [Google Scholar]
- 11.Vergani D, Choudhuri K, Bogdanos D P.et al Pathogenesis of autoimmune hepatitis. Clin Liver Dis 20026439–449. [DOI] [PubMed] [Google Scholar]
- 12.Czaja A J, Manns M P, McFarlane I G.et al Autoimmune hepatitis: the investigational and clinical challenges. Hepatology 2000311194–1200. [DOI] [PubMed] [Google Scholar]
- 13.Pando M, Larriba J, Fernandez G C.et al Paediatric and adult forms of type I autoimmune hepatitis in Argentina: evidence for differential genetic predisposition. Hepatology 1999301374–1380. [DOI] [PubMed] [Google Scholar]
- 14.Marcos Y, Fainboim H A, Capucchio M.et al Two‐locus involvement in the association of human leukocyte antigen with the extrahepatic manifestations of autoimmune chronic active hepatitis. Hepatology 1994191371–1374. [PubMed] [Google Scholar]
- 15.Fainboim L, Marcos Y, Pando M.et al Chronic active autoimmune hepatitis in children. Strong association with a particular HLA‐DR6 (DRB1*1301) haplotype. Hum Immunol 199441146–150. [DOI] [PubMed] [Google Scholar]
- 16.Fainboim L, Pando M, Capucchio M.et al HLA and autoimmune hepatitis. In: Charron D, editor. HLA: genetic diversity of HLA functional and medical implication. Paris: EDK, 1997418–422.
- 17.Donaldson P T, Doherty D G, Hayllar K M.et al Susceptibility to autoimmune chronic active hepatitis: human leukocyte antigens DR4 and A1‐B8‐DR3 are independent risk factors. Hepatology 199113701–706. [PubMed] [Google Scholar]
- 18.Alvarez F, Berg P A, Bianchi F B.et al International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 199931929–938. [DOI] [PubMed] [Google Scholar]
- 19.Napoli J, Bishop A, McGuinness P.et al Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1‐associated cytokines. Hepatology 199624759–765. [DOI] [PubMed] [Google Scholar]
- 20.Morshed S A, Fukuma H, Kimura Y.et al Interferon‐gamma, interleukin (IL)‐2 and IL‐2 receptor expressions in hepatitis C virus‐infected liver. Gastroenterol Jpn 1993559–66. [DOI] [PubMed] [Google Scholar]
- 21.Knodell R, Ishak K G, Black W C.et al Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 19811431–435. [DOI] [PubMed] [Google Scholar]
- 22.Ishak K, Baptista A, Bianchi L.et al Histological grading and staging of chronic hepatitis. J Hepatol 199522696–699. [DOI] [PubMed] [Google Scholar]
- 23.Taylor C R, Shi S R, Chen C.et al Comparative study of antigen retrieval heating methods: Microwave, microwave and pressure cooker, autoclave, and steamer. Biotech Histochem 199671263–270. [DOI] [PubMed] [Google Scholar]
- 24.Jassem W, Koo D D, Cerundolo L.et al Leukocyte infiltration and inflammatory antigen expression in cadaveric and living‐donor livers before transplant. Transplantation 2003752001–2007. [DOI] [PubMed] [Google Scholar]
- 25.Cherñavsky A C, Paladino N, Rubio A E.et al Simultaneous expression of Th1 cytokines and IL‐4 confers severe characteristics to type I autoimmune hepatitis in children. Hum Immunol 200465683–691. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo I, Ikuno N, Omagari K.et al Autoimmune reactivity of sera to hepatocyte plasma membrane in type I autoimmune hepatitis. J Gastroenterol 200035226–234. [DOI] [PubMed] [Google Scholar]
- 27.Mieli‐Vergani G, Vergani D, Jenkins P J.et al Lymphocyte cytotoxicity to autologous hepatocytes in HBs Ag negative chronic active hepatitis. Clin Exp Immunol 19793816–21. [PMC free article] [PubMed] [Google Scholar]
- 28.Galle P R. Apoptosis in liver disease. J Hepatol 199727405–412. [DOI] [PubMed] [Google Scholar]
- 29.Feldmann G. Liver apoptosis. J Hepatol 199726(suppl 2)1–11. [DOI] [PubMed] [Google Scholar]
- 30.Arii S, Imamura M. Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg 2000740–48. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z, Liu Y, Savas L.et al Frequency and distribution of DNA fragmentation as a marker of cell death in chronic liver diseases. Virchows Arch 1997431189–194. [DOI] [PubMed] [Google Scholar]
- 32.Bertolino P, Bowen D G, McCoughan G W.et al Antigen‐specific primary activation of CD8+ T cells within the liver. J Immunol 20011665430–5438. [DOI] [PubMed] [Google Scholar]
- 33.Methal W Z, Juedes A E, Crispe I N. Selective retention of activated CD8+ T cells by the normal liver. J Immunol 19991633202–3210. [PubMed] [Google Scholar]
- 34.Agrati C, Nisi C, Oliva A.et al Lymphocyte distribution and intrahepatic compartmentalization during HCV infection: a main role for MHC‐unrestricted T cells. Arch Immunol Ther Exp 200250307–316. [PubMed] [Google Scholar]
- 35.Crotta S, Stilla A, Wack A.et al Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med 200219535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]