Abstract
Objectives
To assess the safety and cardiopulmonary adaptation to high altitude exposure among patients with coronary artery disease.
Methods
22 patients (20 men and 2 women), mean age 57 (SD 7) years, underwent a maximal, symptom limited exercise stress test in Bern, Switzerland (540 m) and after a rapid ascent to the Jungfraujoch (3454 m). The study population comprised 15 patients after ST elevation myocardial infarction and 7 after a non‐ST elevation myocardial infarction 12 (SD 4) months after the acute event. All patients were revascularised either by percutaneous coronary angioplasty (n = 15) or by coronary artery bypass surgery (n = 7). Ejection fraction was 60 (SD 8)%. β blocking agents were withheld for five days before exercise testing.
Results
At 3454 m, peak oxygen uptake decreased by 19% (p < 0.001), maximum work capacity by 15% (p < 0.001) and exercise time by 16% (p < 0.001); heart rate, ventilation and lactate were significantly higher at every level of exercise, except at maximum exertion. No ECG signs of myocardial ischaemia or significant arrhythmias were noted.
Conclusions
Although oxygen demand and lactate concentrations are higher during exercise at high altitude, a rapid ascent and submaximal exercise can be considered safe at an altitude of 3454 m for low risk patients six months after revascularisation for an acute coronary event and a normal exercise stress test at low altitude.
Keywords: chronic ischaemic heart disease, exercise, exercise testing, rehabilitation, high altitude
Leisure time activities at moderate or high altitude are very popular in mountain areas. An increasing number of recreational facilities with easy access even to high altitudes allows a broad public, including sedentary people, elderly people and patients with various diseases, to be exposed to altitudes beyond 3000 m. In patients with coronary artery disease (CAD), the safety of high altitude exposure has been of concern for a long time. Recommendations to date for exposure to or activities at high altitude in this group of patients are based on empirical rather than scientific data, since only a few reports on high altitude exposure with a very limited number of cardiac patients have been published.1,2,3,4,5
The reduction in the inspired oxygen pressure with increasing altitude leads to several important circulatory changes. These changes lead to an increase in cardiac work and cardiac oxygen consumption),6 which may put cardiac patients at risk, raising concern about the safety of high altitude exposure. In patients with CAD, Wyss et al7 observed a significant decrease in exercise induced coronary flow reserve during inhalation of a hypoxic gas mixture corresponding to an altitude of 2500 m compared with baseline measurements at 450 m. This may indicate that compensatory mechanisms that suffice at low altitude may be exhausted even at moderate altitudes in patients with CAD.
Therefore, we studied cardiopulmonary adaptation to exercise and safety of a rapid but realistic touristic ascent to an altitude of 3454 m, which corresponds to the altitude of the highest located tourist attraction in the Swiss alpine region with easy access by mountain cogwheel railway (Jungfraujoch, Bernese Oberland), visited by > 500 000 people a year.
METHODS
Study population
The study population comprised 22 patients (two women, 20 men), with a mean age of 57 (SD 7) years and a body mass index of 26 (SD 4) kg/m2. All patients had a history of an acute coronary event, which was an ST elevation myocardial infarction in 15 and a non‐ST elevation myocardial infarction in seven patients. During the acute event, mean peak creatine kinase concentration was 65 (SD 91) μg/l, peak troponin I was 124 (SD 239) μg/l and ejection fraction was 58 (SD 11)%. Six patients had three vessel disease, six patients had two vessel disease and 10 patients had one vessel disease. Fifteen patients were treated by percutaneous coronary angioplasty and seven patients by coronary artery bypass grafting. Three vessels with a stenosis < 30% and three vessels with a stenosis < 50% were not revascularised. The culprit lesion was the left anterior descending artery in seven, the circumflex artery in five and the right coronary artery in 10 patients. After the acute event, all patients participated in an 8–12 week ambulatory rehabilitation programme. Twenty two consecutive patients willing to participate were recruited 6–18 months after the acute event (mean 12 (SD 4) months). At study entry, left ventricular systolic function, measured by echocardiography, was normal with an ejection fraction of 60 (SD 8)% and B‐type natriuretic peptide of 50.1 (SD 62.1) pg/ml.
Exclusion criteria were age > 70 years, left ventricular ejection fraction < 45%, an abnormal stress test (chest pain or significant ST segment depression), uncontrolled arterial hypertension (> 160/95 mm Hg at rest), peripheral artery occlusion (> IIb according to the Fontaine classification), functionally relevant valvular disease or significant lung disease (maximum vital capacity or forced expiratory volume in one second < 70% of predicted value).
Study protocol
After an initial stress test for screening, all patients underwent a symptom limited cardiopulmonary exercise stress test at our cardiovascular centre (540 m). β blockers were stopped five days before the test for the whole study period. Within less than three weeks after the first cardiopulmonary exercise stress test, patients started their excursion to the Jungfraujoch at 07 00 and reached the altitude of 3454 m by public transportation at 10 30. The stay at the Jungfraujoch lasted 4 h and included a regular sight seeing programme and a light meal. The cardiopulmonary exercise stress test was performed indoors in a conference room at the tourist complex between 1–3 h after arrival at this altitude.
The patients were informed about the experimental procedures and possible risks related to the present study and provided informed consent. The local ethics committee reviewed and approved the study protocol.
Exercise testing
Cardiopulmonary exercise stress testing with breath by breath gas exchange measurements was performed on a computer controlled, rotational, speed independent bicycle ergometer (Cardiovit CS‐200 Ergo‐Spiro; Schiller AG, Baar, Switzerland). Gases were calibrated each day taking into account the ambient barometric pressure (494 mm Hg). The flow sensor was calibrated before each test. A 12 lead ECG was recorded continuously. Each test consisted of a baseline gas exchange measurement at rest during 1 min, a 3 min reference phase during which patients cycled without a workload and a test phase with a 15 W/min ramp protocol. The following parameters were measured every 2 min: blood pressure, heart rate, subjective rating of perceived exertion (Borg scale8) for dyspnoea and muscle fatigue, and lactate concentration (capillary blood samples from the earlobe; Lactate Pro; Arkray Inc, Kyoto, Japan). Gas exchange parameters were Vo2, carbon dioxide output, tidal volume and breathing rate. From these data, minute ventilation and respiratory exchange ratio (ratio of carbon dioxide output to Vo2) were calculated. Peak Vo2 was defined as the highest Vo2 achieved during the last 30 s of peak exercise. The anaerobic threshold was defined by three criteria: the point after which the respiratory gas exchange ratio becomes 1.0, the V slope method9 and the point at which the ventilatory equivalent for oxygen (ratio of minute ventilation to Vo2) and end tidal oxygen pressure was minimal, followed by a steady increase.
Statistical analysis
All data are expressed as mean (SD). The Wilcoxon signed ranks test was used to calculate p values for comparison of the means. A value of p < 0.05 was considered significant. Data were analysed with SPSS software V.10.0 (SPSS Inc, Chicago, Illinois, USA).
RESULTS
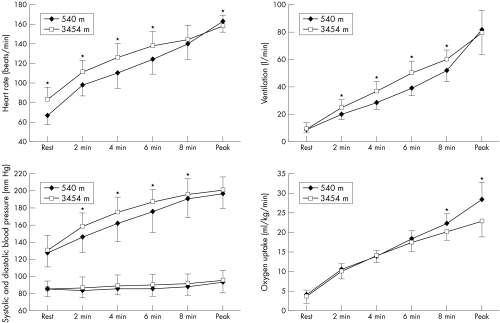
Figure 1 and table 1 show the results for heart rate, blood pressure, ventilation, Vo2, subjective rating of perceived exertion and lactate concentration with cardiopulmonary exercise testing at 540 m and 3454 m.
Figure 1 Results of cardiopulmonary exercise testing in 22 patients with coronary artery disease (CAD) at 540 m (diamonds) and at 3454 m (squares): heart rate, blood pressure, ventilation and oxygen consumption. *p < 0.05.
Table 1 Changes of cardiorespiratory variables during symptom limited exercise test at low (540 m) compared with high altitude (3454 m) (n = 22).
| Variable | Rest | 2 min | 4 min | 6 min | 8 min | Peak |
|---|---|---|---|---|---|---|
| Heart rate (beats/min) | ||||||
| 540 m | 67 (9) | 98 (11) | 111 (15) | 124 (15) | 141 (16) | 163 (11) |
| 3454 m | 83 (13) | 111 (12) | 126 (14) | 138 (14) | 144 (15) | 159 (11) |
| p Value | <0.001 | <0.001 | <0.001 | 0.003 | 0.027 | 0.021 |
| Systolic blood pressure (mm Hg) | ||||||
| 540 m | 128 (17) | 146 (19) | 162 (17) | 176 (24) | 190 (22) | 197 (20) |
| 3454 m | 130 (18) | 158 (16) | 175 (18) | 187 (15) | 196 (18) | 201 (16) |
| p Value | 0.334 | 0.001 | 0.012 | 0.026 | 0.030 | 0.238 |
| Diastolic blood pressure (mm Hg) | ||||||
| 540 m | 85 (9) | 84 (8) | 86 (8) | 86 (9) | 88 (10) | 94 (13) |
| 3454 m | 85 (10) | 86 (13) | 88 (13) | 90 (13) | 90 (12) | 94 (13) |
| p Value | 0.136 | 0.490 | 0.566 | 0.276 | 0.799 | 0.968 |
| Ventilation (l/min) | ||||||
| 540 m | 9 (3) | 20 (4) | 29 (5) | 39 (6) | 52 (8) | 82 (19) |
| 3454 m | 9 (4) | 25 (6) | 37 (7) | 50 (9) | 60 (7) | 78 (16) |
| p Value | 0.395 | 0.003 | <0.001 | <0.001 | 0.006 | 0.265 |
| Oxygen uptake (ml/kg/min) | ||||||
| 540 m | 4.2 (1.1) | 10.5 (1.5) | 14.1 (1.5) | 18.2 (2.2) | 22.3 (2.5) | 28.3 (4.4) |
| 3454 m | 3.6 (1.7) | 10.1 (1.9) | 14.1 (1.7) | 17.5 (2.2) | 20.2 (2.2) | 22.9 (3.9) |
| p Value | 0.266 | 0.305 | 0.741 | 0.126 | 0.004 | <0.001 |
| Lactate (mmol/l) | ||||||
| 540 m | 1.5 (0.4) | 1.5 (0.3) | 1.8 (0.6) | 2.3 (0.9) | 3.6 (1.4) | 7.1 (1.8) |
| 3454 m | 1.7 (0.5) | 1.6 (0.4) | 2.0 (0.6) | 3.4 (1.3) | 4.3 (1.0) | 6.9 (1.5) |
| p Value | 0.024 | 0.082 | 0.022 | <0.001 | 0.058 | 0.715 |
| Perceived exertion rating for leg fatigue | ||||||
| 540 m | NA | 8.7 (1.9) | 10.6 (2.3) | 12.2 (1.6) | 14.0 (1.9) | 16.7 (1.3) |
| 3454 m | NA | 9.9 (1.9) | 12.1 (2.4) | 13.6 (2.3) | 13.9 (1.2) | 16.1 (2.1) |
| p Value | NA | 0.006 | 0.003 | 0.007 | 0.106 | 0.117 |
| Perceived exertion rating for dyspnoea | ||||||
| 540 m | NA | 8.7 (1.3) | 10.5 (2.1) | 12.1 (1.7) | 14.1 (2.4) | 16.7 (1.4) |
| 3454 m | NA | 9.8 (1.7) | 12.5 (2.3) | 14.3 (2.4) | 14.9 (1.1) | 17.4 (1.6) |
| p Value | NA | 0.009 | 0.001 | 0.001 | 0.012 | 0.012 |
Values are presented as mean (SD).
NA, not applicable.
Heart rate
At 3454 m, heart rate at rest was increased by 19% compared with that at low altitude (67 (9) v 83 (13) beats/min, p < 0.001). For every stage of exercise, heart rate was significantly higher at high altitude, except for peak heart rate at maximum effort. At 3454 m, peak heart rate was significantly lower than at 540 m (159 (11) v 163 (11), p = 0.021).
Blood pressure
There was no significant difference in systolic and diastolic blood pressures between 3454 m and 540 m at rest and at maximal exercise. During the other stages, systolic blood pressure was significantly higher at high altitude, whereas diastolic blood pressure did not differ significantly.
Ventilation
At rest and at peak exercise, ventilation did not differ significantly at high and low altitude. During the test, ventilation was significantly increased at high altitude at all stages.
Oxygen uptake
Vo2 increased equally during submaximal exercise at both altitudes. At 3454 m, Vo2 increased less steeply with increasing work load after 6 min at high altitude and at exhaustion peak Vo2 was significantly lower (22.9 (3.9) v 28.3 (4.4) ml/kg/min, p < 0.001). Mean peak Vo2 at low altitude corresponded to 96% of the predicted value (63–123%).
Subjective rating of perceived exertion (Borg scale)
Perceived dyspnoea was significantly higher at all stages of exercise, whereas perceived leg fatigue was significantly different only up to 6 min of exercise.
Lactate
At every stage, lactate concentrations were higher at 3454 m, except for the maximum value. Whereas at the beginning of exercise differences were only marginal, the values diverged clearly after 4 min. At maximal exercise, lactate concentrations were 7.1 (1.8) at 540 m and 6.9 (1.5) mmol/l at 3454 m (p = 0.715).
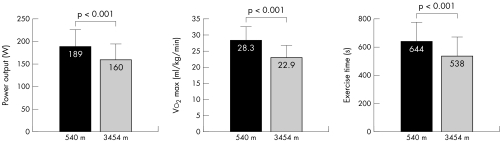
Figure 2 shows the results of cardiopulmonary exercise testing in regard to exercise capacity, Vo2 and exercise time. At exhaustion, maximum power output decreased from 189 to 160 W (−15%, p <0.001), peak Vo2 decreased from 28.3 to 22.9 ml/kg/min (−19%, p < 0.001) and exercise duration decreased from 644 to 538 s (−16%, p < 0.001).
Figure 2 Results of cardiopulmonary exercise testing in 22 patients with coronary artery disease (CAD) at 540 m and at 3454 m: power output, peak Vo2 and exercise time at exhaustion.
All patients tolerated the rapid ascent from the plain up to 3454 m and the 4 h stay without complications. None of the cardiopulmonary stress tests had to be stopped prematurely. No evidence of stress induced ischaemia or of significant arrhythmias was noted during the stress test and recovery.
DISCUSSION
Study findings
To our knowledge, this is the first study of the safety of and cardiopulmonary response to exercise among patients after a myocardial infarction at an altitude of 3454 m. For this reason we selected a patient group at very low risk (condition after coronary revascularisation, normal left ventricular function and no signs of ischaemia during an exercise test at low altitude).
A rapid ascent and a 4 h stay in a real life tourist setting were both well tolerated, and no ischaemia or significant arrhythmia occurred during the stress test. To eliminate the influence of β blockers on the physiological adaptation to high altitude, this drug was stopped at least five days before the first exercise test. This actually adds further value to the safety of the study, since the absence of this drug imposed a higher stress on the cardiovascular system during exercise testing.
It is important to note that the data do not apply to patients at a higher risk than that of the population studied. In particular, patients with reduced left ventricular function, with incomplete revascularisation or less than six months after an acute coronary event constitute a different risk population and such patients may be in danger when exposed to such an altitude. This is especially the case for patients presenting symptoms of heart failure. Furthermore, our study was performed inside a building at room temperature, without exposure to extreme weather conditions. Prolonged outdoor physical activity, with low temperatures or hazardous weather conditions, would impose additional physical stress on such patients. We think that the result of this study will, however, allow a considerable number of patients to be exposed to the higher altitudes to which they had been discouraged to travel.
Risk of high altitude exposure in patients with CAD
For patients with stable CAD, there is concern that exposure to high altitude may elicit ischaemia, increase the risk of arrhythmias or provoke an acute coronary event.
In this study, at submaximal exercise, the rate–pressure product was significantly higher at high altitude at every specific power output stage, due to a higher heart rate and a higher systolic blood pressure, thus imposing a greatly increased myocardial oxygen demand. In addition, sympathetic activation associated with hypoxia10 can cause coronary vasoconstriction in regions with abnormal endothelial vasomotor control7,11,12 and further compromise myocardial oxygen delivery. Even though our patients were revascularised, some coronary artery stenoses remained (three vessels with a stenosis < 30% and three vessels with a stenosis < 50%) that were not revascularised, but none of these caused symptoms or abnormalities on the ECG.
The possibility of an increased risk of high grade ventricular arrhythmias is of great concern. The absence of any arrhythmic event with maximal sympathetic activation during maximal exercise stress testing in combination with increased lactic acid concentrations in our patients can be regarded as reassuring. That our patients had normal or only slightly reduced left ventricular function and were fully revascularised, however, must be kept in mind.
Exercise is also known to be associated with an increased risk of myocardial infarction, particularly after heavy exertion.13,14 Levine et al5 reported from a study with a similar design the occurrence of myocardial infarction in a patient after an exercise stress test at an altitude of 2500 m. Whether high altitude adds to the risk of plaque rupture during exercise is unknown. Changes in inflammatory activation, platelet aggregability and fibrinolytic activity, increased blood pressure and shear forces, changes in the arterial tone and twisting of the arteries during exercise and the role of a haemostatic imbalance as a trigger of acute cardiac events have been discussed as promoters of plaque rupture.15 We do not expect that high altitude would have a major influence on these triggering mechanisms. In contrast, regular physical activity has been shown to protect against plaque rupture13,16,17 and therefore a history of regular physical activity may be a good predictor of an uneventful stay at high altitude.
Blood pressure, heart rate and ventilatory response to high altitude
Systemic blood pressure increases in response to hypoxia during the early stages of altitude adaptation.18 Our patients, some of them with treated hypertension, did not have significantly higher resting systolic blood pressures at high altitude. During exercise, however, their systolic pressure response was significantly increased during all stages, except at the maximum. The enhanced blood pressure response is attributed to a higher excitability of arterial chemoreceptors and a reactive increase of the sympathetic nervous tone. Adequate blood pressure control seems therefore important for high altitude exposure tolerance and safety.
At high altitude, submaximal heart rate and cardiac output can rise as much as 50% above sea level values, whereas the heart's stroke volume remains unchanged.19 This increase of submaximal exercise blood flow to compensate arterial desaturation was also observed in our study population. At 3454 m, heart rate at rest was 19% higher than at 540 m and remained significantly higher except at maximum effort, which was even significantly lower (159 (11) v 163 (11) beats/min, p = 0.021). This effect of hypoxia on peak heart rate has been a matter of debate for a long time. In a review of several studies on maximum heart rate after acute hypoxic exposure, Lundby et al20 have highlighted a possible influence of high altitude on peak heart rate.20 They determined peak heart rate at increasing simulated high altitudes in a dose–response study of healthy young men, showing that peak heart rate decreases with increasing severity of acute hypoxia in a linear manner up to 6300 m.20 The heart rate behaviour of our patients with CAD was similar to that of the healthy subjects in the study of Lundby et al20 and endorses their findings. As a consequence of a decreased peak heart rate in a hypoxic environment, the maximal cardiac output is also reduced, leading to a reduction in maximum oxygen delivery to the working muscles. Below 3100 m, the reduction of peak Vo2 is primarily caused by a decrease in arterial oxygen saturation, whereas at higher altitudes, a reduction of cardiac output is likely to contribute to the limitation of maximum Vo2 as well.20 The decrease in peak heart rate seems to be reinforced after days at high altitude and therefore also depends on duration of hypoxic exposure.
Hyperventilation due to reduced arterial oxygen pressure is the most important and most obvious immediate response to high altitude exposure.21,22 Arrival at altitudes ⩾ 2300 m initiates rapid physiological adjustments to compensate for the “thinner” air with reduction in alveolar oxygen pressure. For any given energy expenditure, ventilation increases proportionately with altitude.23,24 At the same time, due to a shift upwards and to the left of the relationship between lactate and workload,25 lactate concentrations at high altitude are higher at each stage of exercise, which also contributes to exercise hyperventilation.
Impact of high altitude on maximum Vo2
While the atmospheric gas composition of oxygen (20.9%), nitrogen (78%) and inert gases (1.1%) remains the same, the progressive decrease in atmospheric pressure during the ascent (from 760 mm Hg at sea level to 697 mm Hg at 540 m and 494 mm Hg at 3454 m) decreases the ambient partial pressure of oxygen (from 159 mm Hg at sea level to 150 mm Hg at 540 m and 104 mm Hg at 3454 m). The mechanisms compensating for this reduced oxygen supply in a single breath can maintain sufficient oxygen delivery to the peripheral tissues at submaximal effort independently of elevation.26 This was also observed in our patients, who during the first 6 min of exercise had the same Vo2 at 540 m and 3454 m. Thereafter, Vo2 at high altitude began to be restricted and at exhaustion peak Vo2 was 19% lower than at low altitude.
Compared with sea level, small declines in peak Vo2 have been described at an altitude as low as 589 m. Thereafter, arterial desaturation causes peak Vo2 of healthy men and women to decrease at a rate of 7–9% per 1000 m altitude up to an altitude of 6300 m, where aerobic capacity declines at an even more rapid, non‐linear rate.27 With a decrease of peak Vo2 of 6.3% per 1000 m difference in altitude, the reduction of Vo2 in our patients with CAD was even lower than the expected rate for normal people.
Study limitations
The study population consisted only of patients with revascularised stable CAD without a relevant reduction of left ventricular function and a normal exercise stress test at low altitude. Furthermore, the environment during exercise testing was well controlled and activity was strenuous only during the exercise stress test. Under outdoor environmental conditions and sustained physical activity at this altitude, the study might have led to different results. Therefore, advice given to patients in regard to high altitude exposure should be restricted to the patients represented and to the activity they undertook in our study.
Conclusion
Submaximal exercise at high altitude imposes a higher myocardial oxygen demand, ventilatory response and lactate concentrations on patients with CAD than at low altitude. Adaptation to acute high altitude exposure and impact on exercise capacity and Vo2 were the same as described for healthy subjects.
On the basis of our data, patients corresponding to our study population can be allowed exposure to an altitude up to 3454 m for a tourist trip six months after an acute coronary event, provided that a stress test with an appropriate rate–pressure product (at least 25 000 mm Hg · beats/min) is normal and left ventricular function is not compromised. Physical activity at a submaximal level for 3–4 h as in our study can be safely done and a short maximal effort during this time turned out to be safe. If patients want to hike for several hours or if they are going to be exposed to wind, rain, snow or cold, normal exercise capacity should be required (⩾ 100% of the predicted value) and patients should be accustomed to regular strenuous physical activities to minimise the risk of incidents.
ACKNOWLEDGEMENTS
We thank physiotherapists Nicole Kloss, Anja Marti, Renate Ruchti and Susanne Meyer for their care of the patients during their stay at the Jungfraujoch. We greatly appreciated the technical support of Peter Hoppler and his team from Schiller AG in the pressure chamber and at the Jungfraujoch. Special thanks to Benno Schenk for enabling the pilot study at the pressure chamber at the University Hospital Zurich.
References
- 1.Erdmann J, Sun K T, Masar P.et al Effects of exposure to altitude on men with coronary artery disease and impaired left ventricular function. Am J Cardiol 199881266–270. [DOI] [PubMed] [Google Scholar]
- 2.Pokan R, Eber B, Fruhwald F M.et al [Physical activity at intermediate altitude by healthy probands and patients with coronary sclerosis]. Wien Med Wochenschr 1994144121–124. [PubMed] [Google Scholar]
- 3.Agostoni P, Cattadori G, Guazzi M.et al Effects of simulated altitude‐induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med 2000109450–455. [DOI] [PubMed] [Google Scholar]
- 4.Morgan B J. The patient with coronary heart disease at altitude: observations during acute exposure to 3100 meters. J Wilderness Med 19901147–153. [Google Scholar]
- 5.Levine B D, Zuckerman J H, deFilippi C R. Effect of high‐altitude exposure in the elderly: the Tenth Mountain Division study. Circulation 1997961224–1232. [DOI] [PubMed] [Google Scholar]
- 6.McArdle W D, Katch F I, Katch V L. Exercise at medium and high altitude. Exercise physiology: energy, nutrition, and human performance. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2001602–622.
- 7.Wyss C A, Koepfli P, Fretz G.et al Influence of altitude exposure on coronary flow reserve. Circulation 20031081202–1207. [DOI] [PubMed] [Google Scholar]
- 8.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970292–98. [PubMed] [Google Scholar]
- 9.Beaver W L, Wasserman K, Whipp B J. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986602020–2027. [DOI] [PubMed] [Google Scholar]
- 10.Saito M, Mano T, Iwase S.et al Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 1988651548–1552. [DOI] [PubMed] [Google Scholar]
- 11.Gordon J B, Ganz P, Nabel E G.et al Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest 1989831946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werns S W, Walton J A, Hsia H H.et al Evidence of endothelial dysfunction in angiographically normal coronary arteries of patients with coronary artery disease. Circulation 198979287–291. [DOI] [PubMed] [Google Scholar]
- 13.Mittleman M A, Maclure M, Tofler G H.et al Triggering of acute myocardial infarction by heavy physical exertion: protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N Engl J Med 19933291677–1683. [DOI] [PubMed] [Google Scholar]
- 14.Lampert R, Joska T, Burg M M.et al Emotional and physical precipitants of ventricular arrhythmia. Circulation 20021061800–1805. [DOI] [PubMed] [Google Scholar]
- 15.Kullo I J, Edwards W D, Schwartz R S. Vulnerable plaque: pathobiology and clinical implications. Ann Intern Med 19981291050–1060. [DOI] [PubMed] [Google Scholar]
- 16.Albert C M, Mittleman M A, Chae C U.et al Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 20003431355–1361. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere M T, Bersano C, Gnemmi M.et al Exercise‐induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 2002106945–949. [DOI] [PubMed] [Google Scholar]
- 18.Insalaco G, Romano S, Salvaggio A.et al Cardiovascular and ventilatory response to isocapnic hypoxia at sea level and at 5,050 m. J Appl Physiol 1996801724–1730. [DOI] [PubMed] [Google Scholar]
- 19.Klausen K. Cardiac output in man in rest and work during and after acclimatization to 3,800 m. J Appl Physiol 196621609–616. [DOI] [PubMed] [Google Scholar]
- 20.Lundby C, Araoz M, van Hall G. Peak heart rate decreases with increasing severity of acute hypoxia. High Alt Med Biol 20012369–376. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey J A, Reddan W G, Birnbaum M L.et al Effects of acute through life‐long hypoxic exposure on exercise pulmonary gas exchange. Respir Physiol 19711362–89. [DOI] [PubMed] [Google Scholar]
- 22.Lenfant C, Sullivan K. Adaptation to high altitude. N Engl J Med 19712841298–1309. [DOI] [PubMed] [Google Scholar]
- 23.West J B, Hackett P H, Maret K H.et al Pulmonary gas exchange on the summit of Mount Everest. J Appl Physiol 198355678–687. [DOI] [PubMed] [Google Scholar]
- 24.Sutton J R, Reeves J T, Wagner P D.et al Operation Everest II: oxygen transport during exercise at extreme simulated altitude. J Appl Physiol 1988641309–1321. [DOI] [PubMed] [Google Scholar]
- 25.Cerretelli P, Veicsteinas A, Marconi C. Anaerobic metabolism at high altitude: the lactacid mechanism. In: Brendel W, Zink RA, eds. High altitude physiology and medicine. Berlin: Springer, 198594–102.
- 26.Pugh L G, Gill M B, Lahiri S.et al Muscular exercise at great altitudes. J Appl Physiol 196419431–440. [DOI] [PubMed] [Google Scholar]
- 27.Fulco C S, Rock P B, Cymerman A. Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med 199869793–801. [PubMed] [Google Scholar]