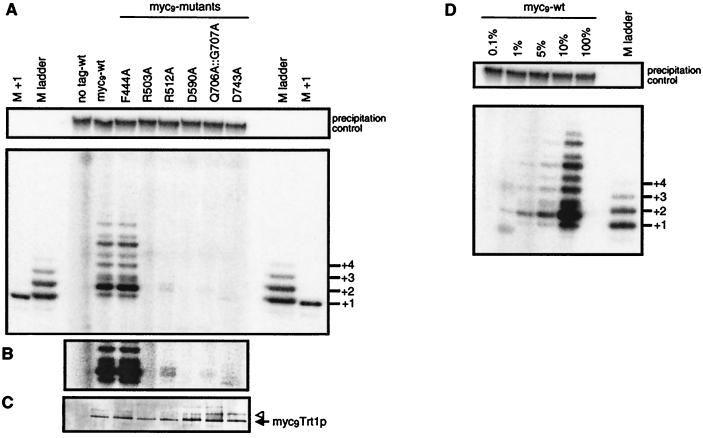
Figure 6.
In vitro telomerase activity of Trt1p mutants. (A) Extracts from S. pombe cells expressing untagged Trt1p, or wild-type or mutant myc9Trt1p fusion protein were immunopurified on monoclonal anti-c-myc antibodies conjugated to agarose beads. Extension of primer oligonucleotide PBoli 14 was assayed as in Fig. 5, where the precipitation control and length marker are also described. (B) A darker exposure of a section better illustrates the primer+2 reaction product for mutant R512A. (C) To test whether equal amounts of myc9Trt1p were bound to the immunoprecipitation beads, the other half of the beads used for the telomerase assay was boiled in Laemmli loading buffer, proteins were resolved on a 6% SDS polyacrylamide minigel, and tagged proteins were revealed by immunoblotting. The arrow and triangle indicate the putative myc-tagged Trt1p species described in the text. (D) Dilution assay to determine the sensitivity of the in vitro telomerase assay. After immunoprecipitation from cells expressing wild-type myc9Trt1p fusion protein, immunoprecipitation beads suspended in TMG(200) were successively diluted and were used in in vitro activity assays as described in A.