Abstract
Objective
To examine whether polymer based coronary stents eluting sirolimus or paclitaxel are equally effective in patients with and without diabetes.
Methods
Systematic review and meta‐analysis by indirect comparison of randomised controlled trials comparing stents eluting sirolimus or paclitaxel with conventional bare metal stents. The overall study population and patients with and without diabetes were analysed separately by using the ratio of incidence rate ratios (RIRR).
Results
The analysis was based on 10 trials (six with sirolimus, four with paclitaxel), 4513 patients (1146 patients with diabetes), 5755 years of follow up, and 2464 events. In patients without diabetes sirolimus eluting stents were superior to paclitaxel eluting stents with respect to in‐stent (RIRR 0.21, 95% confidence interval (CI) 0.10 to 0.48, p < 0.001) and in‐segment restenosis (RIRR 0.47, 95% CI 0.24 to 0.92, p = 0.027), target lesion revascularisation (RIRR 0.54, 95% CI 0.30 to 0.99, p = 0.045), and major adverse cardiac events (RIRR 0.46, 95% CI 0.26 to 0.83, p = 0.010). In patients with diabetes the two drug eluting stents did not differ significantly in any of these end points. Meta‐regression analysis showed a significant difference between patients with and without diabetes (tests for interaction for in‐stent and in‐segment restenosis, p = 0.036 and p = 0.016).
Conclusion
Indirect evidence indicates that sirolimus eluting stents are superior to paclitaxel eluting stents in patients without diabetes but not in patients with diabetes.
Keywords: meta‐analysis, revascularisation, stents, coronary disease, diabetes mellitus
Two drug eluting stents are approved by the US Food and Drug Administration (FDA), a sirolimus and a paclitaxel eluting stent. Both drug eluting stents share a similar stent platform consisting of a stainless steel stent and a non‐biodegradable polymer for controlled drug release. Several randomised controlled trials and meta‐analyses have shown that both drug eluting stents reduce restenosis and the need for repeated revascularisation procedures compared with bare metal stents.1,2,3,4,5,6,7,8,9,10,11
More recently, two large randomised head to head comparisons and a meta‐analysis have shown that sirolimus is superior to paclitaxel in the prevention of restenosis.12,13,14 Open questions remain, however. In particular, it is unclear whether the clinical benefits of these two drug eluting stents are similar across patient groups who differ in terms of underlying cardiovascular risk. Diabetes mellitus is a common and a major risk factor for cardiovascular disease.15,16 Patients with diabetes tend to present with more advanced coronary artery disease, and outcomes after percutaneous coronary intervention (PCI) tend to be poorer than for patients without diabetes.17,18,19 The beneficial effect of drug eluting stents appears attenuated in patients with diabetes compared with patients without diabetes, most probably due to more severe neointimal hyperplasia.20,21 The objective of the present study was to indirectly compare the effects of polymer based sirolimus versus paclitaxel eluting stents and to evaluate whether they are equally effective in the prevention of restenosis in patients with and without diabetes.
METHODS
Literature search and eligibility criteria
We identified all randomised clinical trials that compared the two commercially available, polymer based drug eluting stent systems (the Cypher stent, Cordis, Miami Lakes, Florida, USA, which elutes sirolimus; and the Taxus stent, Boston Scientific, Natick, Massachusetts, USA, which elutes paclitaxel) with bare metal stents. By using Cochrane methods we searched Medline, Embase, and the Cochrane controlled trials register (from inception to April 2004) for relevant studies in any language. Electronic searches were supplemented by manual searching of reference lists, reviews, relevant book chapters, conference abstracts, and specialist journals. We also scrutinised the proceedings of the relevant FDA advisory panels.
We evaluated each trial for inclusion in the meta‐analysis on the basis of five criteria: (1) study design (randomised controlled trial); (2) study population (patients with stable or unstable angina as defined elsewhere22,23 and signs of myocardial ischaemia—patients had to have a new target lesion in a native coronary artery); (3) intervention group (sirolimus or paclitaxel polymer based stent systems); (4) control group (bare metal stent); and (5) length of follow up (at least four months). Two reviewers (CS, SA) independently assessed publications for eligibility, with discrepancies being resolved in consultation with a third reviewer (PD, BM).
Data extraction and outcome measures
Two investigators (CS and SA) independently extracted data, with disagreements resolved by a third reviewer (PD or BM). All relevant publications from a trial were considered, including, for example, early publications describing the study design. Authors from all studies were contacted and asked to check the information extracted from published articles and, where necessary, to provide additional data. Study end points were defined as follows: (1) in‐stent restenosis (stenosis of 50% or greater of the target lesion, confirmed by coronary angiography or intravascular ultrasound); (2) in‐segment restenosis (stenosis of 50% or greater of the target segment, confirmed by coronary angiography or intravascular ultrasound); (3) target lesion revascularisation (coronary artery bypass grafting or repeat PCI procedure at the original lesion site, including the area inside the stent and the 5 mm vessel segments adjacent to it); (4) major adverse cardiac events (MACE) (Q wave and non‐Q wave myocardial infarction, surgical revascularisation (coronary artery bypass graft), percutaneous revascularisation (PCI), or death).
Assessment of methodological quality
Two of us (CS and SA) independently assessed the adequacy of the concealment of allocation of patients to treatment groups and blinding of care providers and research staff ascertaining cardiovascular outcomes. Disagreements were resolved in discussion with a third reviewer (ME).
Statistical analysis
We calculated the incidence rate by dividing the number of events by the number of person years of follow up and separately analysed all patients, patients with diabetes, and patients without diabetes. For each comparison and end point the incidence rate ratio (IRR) was obtained by dividing the incidence in the drug eluting stent group by the incidence in the bare metal stent group. Studies with no outcome events in either group were excluded from the respective analysis. Comparisons with events in only one group were analysed by adding one half to all cells. We combined IRRs in fixed effects meta‐analysis by using inverse variance weighting and calculated the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. We also did standard tests of heterogeneity.24 The numbers of patients needed to be treated with drug eluting rather than bare metal stents to prevent one adverse event was calculated by applying the combined IRRs to the median incidence rate in the bare metal stent group of patients with or without diabetes. In sensitivity analyses we repeated calculations by using random effects models and did tests of funnel plot asymmetry.25 For comparisons between the two drug eluting stent systems we calculated the ratio of IRRs (RIRR) by using a random effects meta‐regression model.26
Crude and adjusted indirect comparisons were performed by fitting random effects meta‐regression models.26 Variables entered in the model were the drug (sirolimus versus paclitaxel), stent strut thickness, study characteristics (dimensions of trial quality and length of angiographic and clinical of follow up), angiographic parameters (length of target lesion, reference vessel diameter, proportion of patients with angiographic follow up, mean duration of use of clopidogrel or ticlopidine, proportion of patients receiving glycoprotein IIb/IIIa antagonists, target artery, American College of Cardiology/American Heart Association lesion classification, proportion of patients with multivessel disease, proportion of patients with stable and unstable angina, and use of direct stenting), and the characteristics of study populations at baseline (mean age, proportion of women, proportion of patients with hypertension or dyslipidaemia, and proportion of smokers). A recent analysis of data from the BENESTENT II (Belgian Netherlands stent) study showed that the inclusion of angiographic follow up increased the number of repeat revascularisations by a factor of 1.6.27,28 In a sensitivity analysis we reanalysed the data with this factor to correct for angiography driven revascularisations. Results are presented as IRRs with 95% confidence intervals (CIs) and numbers needed to treat and 95% CIs. All analyses were performed with Stata version 8.2 (Stata Corporation, College Station, Texas, USA).
RESULTS
Identification of eligible studies
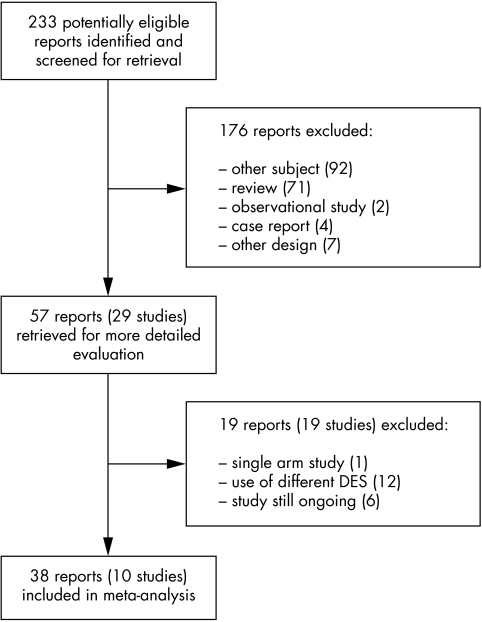
We screened the titles and abstracts of 233 potentially eligible reports, examined the full text of 57 articles reporting on 29 different studies, and identified 10 studies that met our inclusion criteria (fig 1). Additional, unpublished data were obtained for seven trials.1,2,3,5,6,29,30
Figure 1 Identification of eligible randomised controlled trials. DES, drug eluting stents.
Characteristics of trials and patients
Six trials1,2,3,4,8,30 examined the sirolimus and four5,6,7,31 the paclitaxel eluting stent. Trials were of high methodological quality: appropriate methods of allocation concealment were described for all trials and most trials reported analyses according to the intention to treat principle. For one trial the degree of blinding of outcome assessors was unclear.3 In all trials patients with recent acute myocardial infarction or a stenosis of 50% or greater in the left main coronary artery and patients with heart failure were excluded. In all studies except one patients with diabetes constituted a subgroup of the study population.30 Stratified randomisation of patients with and without diabetes was reported in two trials.4,7 Five trials were performed in Europe1,5,8,30,31 and three in North America,3,4,7 and two were multicentre trials performed in Europe and North, Central, and South America.2,6
The 10 trials included a total of 4513 patients, 1146 (25%) patients with and 3367 (75%) patients without diabetes. Table 1 shows the characteristics of the study participants. Patient characteristics were generally comparable across trials.1,2,3,4,5,6,7,8,29,30,31,32,33 The mean age of patients at baseline ranged from 60–67 years. The proportions of women, smokers, and patients with hypertension or dyslipidaemia varied somewhat. Indications for PCI were similar across trials (table 2). There was a tendency towards a smaller mean reference vessel diameter in trials with sirolimus. Mean angiographic follow up and clinical follow up ranged from six to nine months and eight to 24 months, respectively.
Table 1 Characteristics of randomised trials comparing drug eluting stents with bare metal stents.
| Study | Diabetes mellitus | Mean age at baseline (years) | Women (%) | Hypertension (%) | Dyslipidaemia (%) | Smoking (%) | Mean follow up (months) | ||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Angiographic | Clinical | ||||||
| Sirolimus eluting stents | |||||||||
| SIRIUS (2003)4 | 279 | 778 | 62.3 | 29.0 | 68.0 | 74.0 | 20.0 | 8 | 9 |
| E‐SIRIUS (2003)1 | 81 | 271 | 62.3 | 29.3 | 64.0 | 74.0 | 33.0 | 8 | 9 |
| C‐SIRIUS (2004)3 | 24 | 76 | 60.5 | 31.0 | 52.0 | 85.0 | 37.0 | 8 | 9 |
| DIABETES (2005)30 | 160 | 66.6 | 37.5 | 66.3 | 61.3 | 47.5 | 9 | 9 | |
| RAVEL (2002)2 | 44 | 194 | 60.7 | 24.0 | 61.0 | 40.0 | 30.0 | 6 | 12 |
| SES‐SMART (2004)8 | 64 | 193 | 63.6 | 28.4 | 64.7 | 63.0 | 16.3 | 8 | 8 |
| Paclitaxel eluting stents | |||||||||
| TAXUS I (2003)5 | 11 | 49 | 64.9 | 11.4 | 63.9 | 80.3 | 50.8 | 6 | 12 |
| TAXUS II (2003)6, 32, 33 | 76 | 453 | 60.1 | 24.4 | 61.5 | 76.6 | 24.8 | 6 | 24 |
| TAXUS IV (2004)7, 29 | 318 | 996 | 62.5 | 27.9 | 69.8 | 65.3 | 21.8 | 9 | 24 |
| TAXUS VI (2005)31 | 89 | 357 | 62.6 | 23.7 | 57.8 | 71.9 | NA | 9 | 12 |
C‐SIRIUS, Canadian sirolimus coated balloon expandable stent in the treatment of patients with de novo coronary artery lesions; DIABETES, diabetes and sirolimus eluting stent trial; E‐SIRIUS, European sirolimus coated balloon expandable stent in the treatment of patients with de novo coronary artery lesions; NA, not available; RAVEL, randomised study with the sirolimus eluting velocity balloon expandable stent; SES‐SMART, randomised comparison of a sirolimus eluting stent and a standard stent in the prevention of restenosis in small coronary arteries; SIRIUS, sirolimus coated balloon expandable stent in the treatment of patients with de novo coronary artery lesions.
Table 2 Angiographic parameters for studies with sirolimus eluting stents.
| Study | Indications for PCI | Mean RVD (mm) | Mean lesion length (mm) | Target artery (%) | ACC/AHA class (%) | Multivessel disease (%) | Previous MI (%) | AP (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAD | RCA | LCX | A | B1 | B2 | C | Stable | Unstable | ||||||
| Sirolimus trials | ||||||||||||||
| SIRIUS (2003)4 | Stable or unstable AP, signs of myocardial ischaemia | 2.80 | 14.40 | 44 | 31 | 25 | 8 | 36 | 33 | 23 | 42 | 31 | 58 | 53 |
| E‐SIRIUS (2003)1 | Stable or unstable AP, silent ischaemia | 2.55 | 15.00 | 56 | 21 | 23 | NA | NA | NA | NA | 36 | 42 | NA | 33 |
| C‐SIRIUS (2004)3 | Stable or unstable AP, silent ischaemia | 2.63 | 13.60 | 36 | 41 | 23 | NA | NA | 59 | 40 | 42 | 12 | 51 | |
| DIABETES (2005)30 | Symptoms or objective evidence of ischaemia | 2.34 | 15.00 | 41 | 37 | 22 | NA | NA | 80 | 65 | 37 | NA | NA | |
| RAVEL (2002)2 | Stable or unstable AP, silent ischaemia | 2.62 | 9.58 | 50 | 27 | 23 | 6 | 37 | 57 | 0 | NA | 36 | 39 | 50 |
| SES‐SMART (2004)8 | ACS, stable AP, silent myocardial ischaemia as shown by exercise stress test | 2.20 | 11.84 | 28 | 16 | 30 | 25 | 47 | 24 | 4 | 65 | NA | NA | NA |
| Paclitaxel trials | ||||||||||||||
| TAXUS I (2003)5 | Stable or unstable AP or silent ischaemia | 2.97 | 11.30 | 40 | 30 | 30 | 23 | 41 | 36 | 0 | NA | 28 | NA | NA |
| TAXUS II (2003)6, 32, 33 | Stable or unstable AP or silent ischaemia | 2.75 | 10.48 | 45 | 36 | 19 | NA | NA | NA | NA | NA | 40 | 60 | 34 |
| TAXUS IV (2004)7, 29 | Stable or unstable angina or provokable ischaemia | 2.75 | 13.40 | 41 | 31 | 28 | NA | NA | NA | NA | NA | 30 | NA | 34 |
| TAXUS VI (2005)31 | Stable or unstable AP or silent ischaemia | 2.78 | 20.62 | NA | NA | 28 | 56 | NA | NA | NA | NA | |||
ACC, American College of Cardiology; ACS, acute coronary syndrome; AHA, American Heart Association; AP, angina pectoris; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCA, right coronary artery; RVD, reference vessel diameter.
Outcomes
Table 3 shows IRRs from individual trials for the four outcomes analysed and combined rate ratios from meta‐analyses. Table 4 and table 5 show the same data for patients with and without diabetes. Figure 2 presents combined results from meta‐analyses for all patients, and fig 3 shows these results separately for patients with and without diabetes. For some trials and outcomes separate data on patients with and without diabetes were not available, which meant that the number of trials that contributed to a given analysis varied. Crude and adjusted RIRR comparing sirolimus versus paclitaxel eluting stents were closely similar, and crude results are therefore presented throughout.
Table 3 Incidence rate ratios (IRRs) from trials of sirolimus and paclitaxel eluting stents and ratio of incidence rate ratios (RIRR) comparing sirolimus with paclitaxel in all patients.
| Study | IRR (95% confidence interval) | |||
|---|---|---|---|---|
| In‐stent restenosis | In‐segment restenosis | TLR | MACE | |
| Sirolimus trials | ||||
| SIRIUS (2003)4 | 0.09 (0.05 to 0.16) | 0.24 (0.16 to 0.36) | 0.24 (0.15 to 0.39) | 0.38 (0.26 to 0.55) |
| E‐SIRIUS (2003)1 | 0.09 (0.04 to 0.22) | 0.14 (0.07 to 0.28) | 0.19 (0.09 to 0.43) | 0.35 (0.19 to 0.65) |
| C‐SIRIUS (2004)3 | 0.02 (0.001 to 0.40) | 0.04 (0.01 to 0.32) | 0.22 (0.05 to 1.03) | 0.22 (0.05 to 1.03) |
| DIABETES (2005)30 | 0.15 (0.06 to 0.39) | 0.22 (0.10 to 0.47) | 0.24 (0.10 to 0.59) | 0.31 (0.15 to 0.66) |
| RAVEL (2002)2 | 0.02 (0.001 to 0.26) | 0.02 (0.001 to 0.26) | 0.02 (0.001 to 0.29) | 0.20 (0.09 to 0.44) |
| SES‐SMART (2004)8 | 0.10 (0.04 to 0.23) | 0.18 (0.10 to 0.34) | 0.33 (0.16 to 0.70) | 0.30 (0.16 to 0.57) |
| Combined IRR | 0.10 (0.07 to 0.14) | 0.20 (0.15 to 0.26) | 0.24 (0.17 to 0.33) | 0.33 (0.25 to 0.42) |
| Heterogeneity* | 0.0%, p = 0.63 | 33.6%, p = 0.18 | 0.0%, p = 0.51 | 0.0%, p = 0.78 |
| Paclitaxel trials | ||||
| TAXUS I (2003)5 | 0.14 (0.01 to 2.67) | NA | 0.14 (0.01 to 2.77) | 0.25 (0.03 to 2.24) |
| TAXUS II (2003)6, 32, 33 | 0.33 (0.19 to 0.55) | 0.18 (0.09 to 0.36) | 0.27 (0.13 to 0.53) | 0.58 (0.38 to 0.86) |
| TAXUS IV (2004)7, 29 | 0.23 (0.13 to 0.39) | 0.30 (0.19 to 0.47) | 0.32 (0.22 to 0.46) | 0.56 (0.41 to 0.78) |
| TAXUS VI (2005)31 | 0.28 (0.17 to 0.46) | 0.35 (0.22 to 0.54) | 0.42 (0.25 to 0.72) | 0.73 (0.48 to 1.12) |
| Combined IRR | 0.27 (0.20 to 0.37) | 0.29 (0.22 to 0.39) | 0.33 (0.25 to 0.44) | 0.60 (0.48 to 0.75) |
| Heterogeneity* | 0.0%, p = 0.77 | 16.3%, p = 0.30 | 0.0%, p = 0.67 | 0.0%, p = 0.65 |
| RIRR (sirolimus v paclitaxel) | 0.35 (0.21 to 0.57) p<0.001 | 0.68 (0.45 to 1.01) p = 0.057 | 0.71 (0.46 to 1.09) p = 0.120 | 0.54 (0.39 to 0.76) p<0.001 |
*I2, test of heterogeneity.
MACE, major adverse cardiac events; TLR, target lesion revascularisation.
Table 4 IRRs from trials of sirolimus and paclitaxel eluting stents and RIRR comparing sirolimus with paclitaxel in patients without diabetes.
| Study | IRR (95% confidence interval) | |||
|---|---|---|---|---|
| In‐stent restenosis | In‐segment restenosis | TLR | MACE | |
| Sirolimus trials | ||||
| SIRIUS (2003)4 | 0.05 (0.02 to 0.14) | 0.20 (0.11 to 0.34) | 0.21 (0.11 to 0.40) | 0.39 (0.25 to 0.62) |
| E‐SIRIUS (2003)1 | 0.09 (0.03 to 0.24) | 0.13 (0.05 to 0.30) | 0.20 (0.08 to 0.52) | 0.42 (0.20 to 0.85) |
| C‐SIRIUS (2004)3 | 0.04 (0.002 to 0.68) | 0.03 (0.002 to 0.54) | 0.13 (0.02 to 1.00) | 0.13 (0.02 to 1.00) |
| RAVEL (2004)2 | 0.02 (0.001 to 0.37) | 0.02 (0.001 to 0.37) | 0.02 (0.001 to 0.41) | 0.20 (0.08 to 0.53) |
| SES‐SMART (2002)8 | NA | 0.11 (0.04 to 0.28) | NA | NA |
| Combined IRR | 0.06 (0.03 to 0.12) | 0.15 (0.10 to 0.22) | 0.19 (0.11 to 0.31) | 0.35 (0.25 to 0.50) |
| Heterogeneity* | 0.0%, p = 0.77 | 8.8%, p = 0.36 | 0.0%, p = 0.52 | 0.0%, p = 0.44 |
| Paclitaxel trials | ||||
| TAXUS I (2003)5 | NA | NA | 0.16 (0.01 to 3.13) | 0.28 (0.03 to 2.53) |
| TAXUS II (2003)6, 32, 33 | NA | 0.08 (0.02 to 0.24) | 0.29 (0.14 to 0.61) | NA |
| TAXUS IV (2004)7, 29 | 0.25 (0.13 to 0.47) | 0.35 (0.20 to 0.59) | 0.30 (0.19 to 0.48) | NA |
| TAXUS VI (2004)31 | 0.30 (0.17 to 0.53) | 0.39 (0.24 to 0.64) | 0.49 (0.27 to 0.88) | 0.80 (0.49 to 1.29) |
| Combined IRR | 0.28 (0.18 to 0.42) | 0.32 (0.23 to 0.45) | 0.35 (0.25 to 0.48) | 0.76 (0.48 to 1.22) |
| Heterogeneity* | 0.0%, p = 0.67 | 69.2%, p = 0.04 | 0.0%, p = 0.53 | 0.0%, p = 0.37 |
| RIRR (sirolimus v paclitaxel) | 0.21 (0.10 to 0.48) p<0.001 | 0.47 (0.24 to 0.92) p = 0.027 | 0.54 (0.30 to 0.99) p = 0.045 | 0.46 (0.26 to 0.83) p = 0.010 |
*I2, test of heterogeneity.
Table 5 IRRs from trials of sirolimus and paclitaxel eluting stents and RIRR comparing sirolimus with paclitaxel in patients with diabetes.
| Study | IRR (95% confidence interval) | |||
|---|---|---|---|---|
| In‐stent restenosis | In‐segment restenosis | TLR | MACE | |
| Sirolimus trials | ||||
| SIRIUS (2003)4 | 0.17 (0.08 to 0.37) | 0.35 (0.20 to 0.62) | 0.31 (0.15 to 0.64) | 0.37 (0.19 to 0.70) |
| E‐SIRIUS (2003)1 | 0.13 (0.03 to 0.57) | 0.19 (0.06 to 0.64) | 0.21 (0.05 to 0.91) | 0.27 (0.08 to 0.94) |
| C‐SIRIUS (2004)3 | 0.06 (0.003 to 1.02) | 0.13 (0.02 to 1.00) | 1.00 (0.06 to 15.99) | 1.00 (0.06 to 15.99) |
| DIABETES (2005)30 | 0.15 (0.06 to 0.39) | 0.22 (0.10 to 0.47) | 0.24 (0.10 to 0.59) | 0.31 (0.15 to 0.66) |
| RAVEL (2002)2 | 0.06 (0.003 to 0.97) | 0.06 (0.003 to 0.97) | 0.07 (0.004 to 1.19) | 0.22 (0.05 to 0.98) |
| SES‐SMART (2004)8 | NA | 0.39 (0.17 to 0.91) | NA | NA |
| Combined IRR | 0.15 (0.09 to 0.25) | 0.28 (0.20 to 0.41) | 0.27 (0.16 to 0.45) | 0.33 (0.21 to 0.51) |
| Heterogeneity* | (0.0%, p = 0.92) | (0.0%, p = 0.59) | (0.0%, p = 0.73) | (0.0%, p = 0.89) |
| Paclitaxel trials | ||||
| TAXUS I (2003)5 | NA | NA | 0 events | 0 events |
| TAXUS II (2003)6, 32, 33 | NA | 0.07 (0.004 to 1.17) | 0.16 (0.02 to 1.25) | NA |
| TAXUS IV (2004)7, 29 | 0.16 (0.05 to 0.48) | 0.18 (0.07 to 0.49) | 0.36 (0.19 to 0.70) | NA |
| TAXUS VI (2005)31 | 0.20 (0.05 to 0.68) | 0.23 (0.08 to 0.66) | 0.20 (0.04 to 0.90) | 0.55 (0.21 to 1.43) |
| Combined IRR | 0.18 (0.08 to 0.40) | 0.19 (0.09 to 0.38) | 0.31 (0.18 to 0.56) | 0.55 (0.21 to 1.43) |
| Heterogeneity* | 0.0%, p = 0.80 | 0.0%, p = 0.73 | 0.0%, p = 0.62 | NA |
| RIRR (sirolimus v paclitaxel) | 0.82 (0.31 to 2.18) p = 0.694 | 1.51 (0.68 to 3.33) p = 0.312 | 0.86 (0.40 to 1.86) p = 0.703 | 0.60 (0.21 to 1.71) p = 0.336 |
*I2, test of heterogeneity.
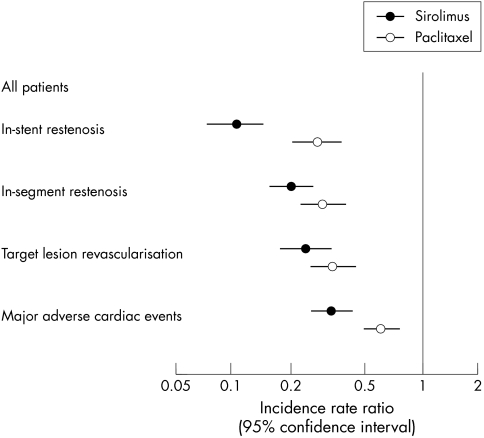
Figure 2 Effect of DES with sirolimus and paclitaxel compared with bare metal stents on the risks of restenosis, revascularisation, or adverse events. Combined estimates from meta‐analyses of randomised controlled trials.
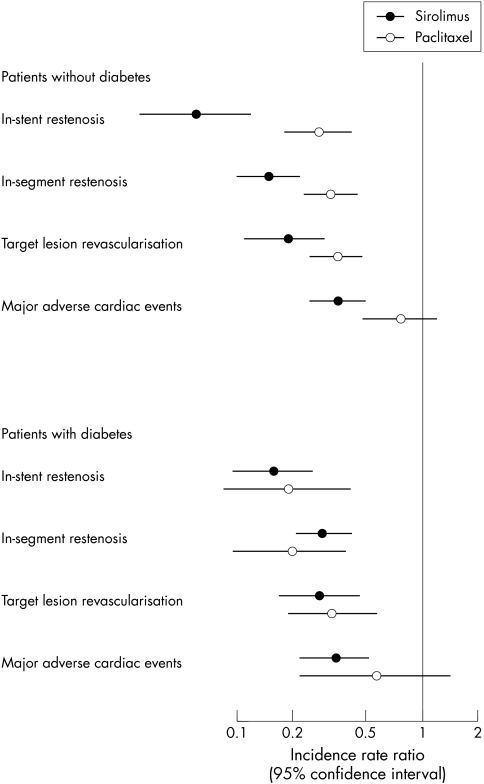
Figure 3 Effect of DES with sirolimus and paclitaxel in patients with and without diabetes.
Restenosis
Overall, analyses were based on 604 episodes of in‐stent restenosis and 657 episodes of in‐segment restenosis. Compared with bare metal stents, drug eluting stents were associated with substantial reductions in the risk of restenosis in all trials reporting this outcome, but reductions were more pronounced with sirolimus than with paclitaxel eluting stents. The combined IRRs for in‐stent and in‐segment restenosis were 0.10 (95% CI 0.07 to 0.14) and 0.20 (95% CI 0.15 to 0.26), respectively, with sirolimus eluting stents, and 0.27 (95% CI 0.20 to 0.37) and 0.29 (95% CI 0.22 to 0.39) with paclitaxel eluting stents. Of note, heterogeneity between study results in these two meta‐analyses was entirely attributable to random variation (I2 = 0%). These results translated into an RIRR of 0.35 (95% CI 0.21 to 0.57) for in‐stent restenosis indicating that, compared with paclitaxel, sirolimus eluting stents led to a reduction in incidence by 65%. The corresponding RIRR for in‐segment restenosis was 0.68 (95% CI 0.45 to 1.01). These differences in the efficacy of preventing restenosis between the two stent systems were attributable to lower rates of restenosis with sirolimus compared with paclitaxel eluting stents in patients without diabetes, whereas results were comparable in patients with diabetes (tables 4 and 5, fig 3). Meta‐regression analysis showed a significant difference between patients with and without diabetes (tests for interaction for in‐stent and in‐segment restenosis, p = 0.036 and p = 0.016).
Revascularisation
Overall, analyses were based on 522 target lesion revascularisations. Compared with bare metal stents, drug eluting stents were associated with a substantial reduction in the risk of revascularisation, but reductions were more pronounced with sirolimus than with paclitaxel eluting stents (RIRR 0.71, 95% CI 0.46 to 1.09). This difference was also more pronounced in patients without diabetes (RIRR 0.54, 95% CI 0.30 to 0.99) than in patients with diabetes (RIRR 0.86, 95% CI 0.40 to 1.86), although the formal test for interaction did not reach conventional levels of significance (p = 0.36). Results were closely similar when correcting for angiography driven revascularisations.
Major adverse cardiac events
Analyses were based on 681 MACE, including 148 myocardial infarctions, and 41 deaths. The TAXUS trialists included stent thrombosis in their definition of MACE (17 events). Reductions were also more pronounced with sirolimus eluting stents than with paclitaxel stents (RIRR 0.54, 95% CI 0.39 to 0.76), and the difference between the two types of drug eluting stents was more pronounced in patients without diabetes (RIRR 0.46, 95% CI 0.26 to 0.83) than in patients with diabetes (RIRR 0.60, 95% CI 0.21 to 1.71, test for interaction p = 0.68).
Numbers needed to treat to prevent one event
Table 6 shows the estimated numbers of patients needed to treat with drug eluting rather than bare metal stents to prevent one outcome event. Numbers needed to treat were lowest for sirolimus eluting stents in patients with diabetes, followed by paclitaxel eluting stents in patients with diabetes, sirolimus eluting stents in patients without diabetes, and paclitaxel eluting stents in patients without diabetes. For one end point (MACE) the CI for the IRR of paclitaxel eluting stents was compatible with benefit and harm. We accounted for this by calculating numbers needed to benefit (corresponding to the lower limit of the CI) and numbers needed to harm (corresponding to the upper limit of the CI).34
Table 6 Number of patients needed to treat during one year to prevent one adverse event.
| Number needed to treat (95% confidence interval) | ||||
|---|---|---|---|---|
| In‐stent restenosis | In‐segment restenosis | TLR | MACE | |
| Sirolimus eluting stent | ||||
| Patients without diabetes | 2.4 (2.3 to 2.6) | 2.6 (2.5 to 2.9) | 6.5 (5.9 to 7.6) | 6.2 (5.3 to 8.0) |
| Patients with diabetes | 1.6 (1.5 to 1.9) | 1.9 (1.7 to 2.3) | 5.5 (4.8 to 7.3) | 4.0 (3.4 to 5.5) |
| Paclitaxel eluting stent | ||||
| Patients without diabetes | 3.2 (2.8 to 3.9) | 3.3 (2.9 to 4.0) | 8.1 (7.0 to 10.1) | 16.7 (NNTB 7.7 to NNTH 18.2)* |
| Patients with diabetes | 1.7 (1.5 to 2.3) | 1.7 (1.5 to 2.2) | 5.8 (4.9 to 9.1) | 6.0 (NNTB 3.4 to NNTH 6.3)* |
*95% confidence interval includes benefit and harm (number of patients needed to treat to prevent one event (NNTB); number of patients needed to treat to harm one patient (NNTH)).34
DISCUSSION
The indirect comparisons presented here indicate that sirolimus eluting stents are superior to paclitaxel eluting stents, and that the difference in the effectiveness between the two drug eluting stent systems is clearly evident in patients without diabetes but less certain in patients with diabetes. Calculations of numbers needed to treat show that, compared with sirolimus eluting stents, about 10 additional patients without diabetes have to be treated with paclitaxel eluting stents to prevent one MACE.
Strengths and limitations
Our review was based on a comprehensive literature search and included assessments of trial quality and a substantial amount of additional information supplied by the original investigators. Although most trials included in this analysis were not designed to examine the effectiveness of drug eluting stents in patients with and without diabetes, randomisation was stratified according to the presence or absence of diabetes in some studies,4,31 and all studies prospectively recorded outcomes according to standardised definitions. Indirect comparisons between sirolimus and paclitaxel eluting stents were appropriate because trials were of high methodological quality and had enrolled similar patient populations. Indeed, results were robust when adjusted for study characteristics and patient characteristics at baseline. We acknowledge that such comparisons are observational in nature and therefore have to be interpreted with caution. Only 10 trials were identified and average follow up was relatively short, which means that there was limited power to detect or exclude differences in effectiveness for rarer but clinically relevant end points, including myocardial infarction, stent thrombosis, and death.
The effect of drug eluting stents on MACE was mainly due to a reduction of revascularisation procedures. As Babapulle et al9 pointed out, the clinical significance of these additional revascularisation procedures is unclear because angiography was done routinely in these trials, at least in a proportion of the study population. Angiographic follow up may influence the rate of revascularisation, especially in patients with diabetes and autonomic neuropathy.35 The impact of angiography on revascularisation rates has recently been quantified.28 When we used these estimates to correct incidences for angiography driven revascularisation, results were not materially altered. We could not examine the influence of glycaemic control on the rate of restenosis and revascularisation; although a protective effect of optimised glycaemic control in patients with diabetes has been shown, no detailed information on glycaemic control was available for the trials we analysed.36
Results in context with other studies
Two meta‐analyses have shown that the presence of diabetes is a risk factor for restenosis, both with drug eluting and bare metal stents.21,37 Our findings confirm these results: diabetes clearly remains a risk factor for restenosis in the drug eluting stent era. Methodological research has shown that indirect comparisons adjusted at the aggregate level usually agree with the results of head to head randomised trials.38 In this study overall results were indeed closely similar to those reported in a recent meta‐analysis of six head to head trials.14 Data from head to head comparisons in patients with diabetes are, however, more limited, and results are more heterogeneous. The large REALITY trial showed no overall difference in restenosis rates between the two stent systems, although sirolimus eluting stents appeared to be superior in patients without diabetes.39 The SIRTAX trial, in contrast, found the sirolimus eluting stent to be superior overall, with a more pronounced reduction of the rate of restenosis and revascularisation in patients with diabetes than in patients without diabetes.12 In both trials the number of patients with diabetes was relatively small, and formal tests of interaction were non‐significant. Lastly, the ISAR‐DIABETES trial in 250 patients with diabetes showed larger reductions in angiographic restenosis (p = 0.03) and target lesion revascularisation (p = 0.13) with sirolimus than with paclitaxel.13
Possible mechanisms
In‐stent restenosis results from neointimal hyperplasia, and the pharmacological inhibition of vascular smooth muscle proliferation by local drug delivery has proved effective in reducing restenosis and thus repeat revascularisation procedures.40,41 The biological mechanisms of action differ between paclitaxel and sirolimus: paclitaxel treated cells form abnormally stable and non‐functional microtubules, which inhibit cellular replication and proliferation.42 In contrast, sirolimus is a macrocyclic lactone that inhibits cytokine mediated and growth factor induced proliferation of smooth muscle cells and has immunoregulatory and anti‐inflammatory properties.43,44,45 One would expect these properties to be particularly beneficial in diabetic atherosclerosis, which is characterised by increased inflammatory markers.46 On the other hand, treatment of human platelets with sirolimus has been shown to result in enhanced agonist induced platelet aggregation and secretion.47 The more complex and advanced nature of lesions in patients with diabetes may interact with the biological mechanisms of action of both drugs but hamper effects of sirolimus more than effects of paclitaxel. Differences in local drug concentrations may also have a role: both drugs are highly lipophilic but different protein binding characteristics mean that sirolimus is distributed evenly through the vessel wall, whereas paclitaxel remains primarily subintimal.48 These distribution patterns and tissue residence time may be modified in atherosclerotic lesions of patients with diabetes. It is also possible that differences in the doses of the two drugs or differences in concomitant medications have a role.
Stents with thinner struts elicit less angiographic and clinical restenosis than stents with thicker struts.49,50 Differences in strut thickness can therefore have affected indirect comparisons. This is, however, unlikely because differences were small (140 and 130 μm for the sirolimus and the paclitaxel eluting stent, respectively). Event rates tended to be somewhat higher in the bare metal stent groups of the sirolimus trials than in the corresponding groups of the paclitaxel trials. If the relative reduction in restenosis risk strongly depended on the control group risk, this can partly explain the superior efficacy observed for sirolimus eluting stents. This is unlikely for several reasons. Recent head to head trials in patient populations that differed in terms of underlying risk consistently showed that sirolimus is superior to paclitaxel.14 Moreover, methodological research has shown that the relative reductions in risk associated with medical interventions tend be constant across patient populations with different underlying risks.51
Conclusions
This systematic review and meta‐analysis shows substantial reductions in restenosis and revascularisation rates with the two widely used polymer based drug eluting stents, in both patients with and patients without diabetes. Sirolimus eluting stents appear more effective than paclitaxel eluting stents in patients without diabetes, whereas efficacy appears to be comparable in patients with diabetes. We submit that a collaborative meta‐analysis based on individual patient data should be performed, which addresses the question whether the effectiveness of the two stents differs across patient groups with and without diabetes and, more in general, between patients at higher or lower risk of complications.
ACKNOWLEDGEMENTS
SA is supported by grants from Novo Nordisk, Roche Diagnostics, and GlaxoSmithKline. We are grateful to the trialists who provided additional data and checked the data we extracted from their publications.
Abbreviations
BENESTENT II - Belgian Netherlands stent II
CI - confidence interval
FDA - Food and Drug Administration
IRR - incidence rate ratio
MACE - major adverse cardiac events
PCI - percutaneous coronary intervention
RIRR - ratio of incidence rate ratios
Footnotes
Competing interests: none declared.
References
- 1.Schofer J, Schluter M, Gershlick A H.et al Sirolimus‐eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double‐blind, randomised controlled trial (E‐SIRIUS). Lancet 20033621093–1099. [DOI] [PubMed] [Google Scholar]
- 2.Morice M C, Serruys P W, Sousa J E.et al A randomized comparison of a sirolimus‐eluting stent with a standard stent for coronary revascularization. N Engl J Med 20023461773–1780. [DOI] [PubMed] [Google Scholar]
- 3.Schampaert E, Cohen E A, Schluter M.et al The Canadian study of the sirolimus‐eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C‐SIRIUS). J Am Coll Cardiol 2004431110–1115. [DOI] [PubMed] [Google Scholar]
- 4.Moses J W, Leon M B, Popma J J.et al Sirolimus‐eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 20033491315–1323. [DOI] [PubMed] [Google Scholar]
- 5.Grube E, Silber S, Hauptmann K E.et al TAXUS I: six‐ and twelve‐month results from a randomized, double‐blind trial on a slow‐release paclitaxel‐eluting stent for de novo coronary lesions. Circulation 200310738–42. [DOI] [PubMed] [Google Scholar]
- 6.Colombo A, Drzewiecki J, Banning A.et al Randomized study to assess the effectiveness of slow‐ and moderate‐release polymer‐based paclitaxel‐eluting stents for coronary artery lesions. Circulation 2003108788–794. [DOI] [PubMed] [Google Scholar]
- 7.Stone G W, Ellis S G, Cox D A.et al A polymer‐based, paclitaxel‐eluting stent in patients with coronary artery disease. N Engl J Med 2004350221–231. [DOI] [PubMed] [Google Scholar]
- 8.Ardissino D, Cavallini C, Bramucci E.et al Sirolimus‐eluting vs uncoated stents for prevention of restenosis in small coronary arteries: a randomized trial. JAMA 20042922727–2734. [DOI] [PubMed] [Google Scholar]
- 9.Babapulle M N, Joseph L, Belisle P.et al A hierarchical Bayesian meta‐analysis of randomised clinical trials of drug‐eluting stents. Lancet 2004364583–591. [DOI] [PubMed] [Google Scholar]
- 10.Hill R A, Dundar Y, Bakhai A.et al Drug‐eluting stents: an early systematic review to inform policy. Eur Heart J 200425902–919. [DOI] [PubMed] [Google Scholar]
- 11.Indolfi C, Pavia M, Angelillo I F. Drug‐eluting stents versus bare metal stents in percutaneous coronary interventions (a meta‐analysis). Am J Cardiol 2005951146–1152. [DOI] [PubMed] [Google Scholar]
- 12.Windecker S, Remondino A, Eberli F R.et al Sirolimus‐eluting and paclitaxel‐eluting stents for coronary revascularization. N Engl J Med 2005353653–662. [DOI] [PubMed] [Google Scholar]
- 13.Dibra A, Kastrati A, Mehilli J.et al Paclitaxel‐eluting or sirolimus‐eluting stents to prevent restenosis in diabetic patients. N Engl J Med 2005353663–670. [DOI] [PubMed] [Google Scholar]
- 14.Kastrati A, Dibra A, Eberle S.et al Sirolimus‐eluting stents vs paclitaxel‐eluting stents in patients with coronary artery disease: meta‐analysis of randomized trials. JAMA 2005294819–825. [DOI] [PubMed] [Google Scholar]
- 15.Kannel W B, McGee D L. Diabetes and cardiovascular disease: the Framingham study. JAMA 19792412035–2038. [DOI] [PubMed] [Google Scholar]
- 16.Stamler J, Vaccaro O, Neaton J D.et al Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care 199316434–444. [DOI] [PubMed] [Google Scholar]
- 17.Carrozza J P, Jr, Kuntz R E, Fishman R F.et al Restenosis after arterial injury caused by coronary stenting in patients with diabetes mellitus. Ann Intern Med 1993118344–349. [DOI] [PubMed] [Google Scholar]
- 18.Stein B, Weintraub W S, Gebhart S P.et al Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation 199591979–989. [DOI] [PubMed] [Google Scholar]
- 19.Detre K M, Guo P, Holubkov R et a l. Coronary revascularization in diabetic patients: a comparison of the randomized and observational components of the bypass angioplasty revascularization investigation (BARI). Circulation 199999633–640. [DOI] [PubMed] [Google Scholar]
- 20.Scheen A J, Warzee F. Diabetes is still a risk factor for restenosis after drug‐eluting stent in coronary arteries. Diabetes Care 2004271840–1841. [DOI] [PubMed] [Google Scholar]
- 21.Scheen A J, Warzee F, Legrand V M. Drug‐eluting stents: meta‐analysis in diabetic patients. Eur Heart J 2004252167–2168. [DOI] [PubMed] [Google Scholar]
- 22.Campeau L. Grading of angina pectoris [letter]. Circulation 197654522–523. [PubMed] [Google Scholar]
- 23.Hamm C W, Braunwald E. A classification of unstable angina revisited. Circulation 2000102118–122. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J P, Thompson S G. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002211539–1558. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey S G, Schneider M.et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997315629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson S G, Sharp S J. Explaining heterogeneity in meta‐analysis: a comparison of methods. Stat Med 1999182693–2708. [DOI] [PubMed] [Google Scholar]
- 27.Serruys P W, van Hout B, Bonnier H.et al Randomised comparison of implantation of heparin‐coated stents with balloon angioplasty in selected patients with coronary artery disease (Benestent II). Lancet 1998352673–681. [DOI] [PubMed] [Google Scholar]
- 28.Van Hout B A, Serruys P W, Lemos P A.et al One year cost effectiveness of sirolimus eluting stents compared with bare metal stents in the treatment of single native de novo coronary lesions: an analysis from the RAVEL trial. Heart 200591507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone G W, Ellis S G, Cox D A.et al One‐year clinical results with the slow‐release, polymer‐based, paclitaxel‐eluting TAXUS stent: the TAXUS‐IV trial. Circulation 20041091942–1947. [DOI] [PubMed] [Google Scholar]
- 30.Sabate M, Jimenez‐Quevedo P, Angiolillo D J.et al Randomized comparison of sirolimus‐eluting stent versus standard stent for percutaneous coronary revascularization in diabetic patients: the diabetes and sirolimus‐eluting stent (DIABETES) trial. Circulation 20051122175–2183. [DOI] [PubMed] [Google Scholar]
- 31.Dawkins K D, Grube E, Guagliumi G.et al Clinical efficacy of polymer‐based paclitaxel‐eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug‐eluting stents in contemporary clinical practice. Circulation 20051123306–3313. [DOI] [PubMed] [Google Scholar]
- 32.Serruys P W, Degertekin M, Tanabe K.et al Vascular responses at proximal and distal edges of paclitaxel‐eluting stents: serial intravascular ultrasound analysis from the TAXUS II trial. Circulation 2004109627–633. [DOI] [PubMed] [Google Scholar]
- 33.Tanabe K, Serruys P W, Degertekin M.et al Chronic arterial responses to polymer‐controlled paclitaxel‐eluting stents: comparison with bare metal stents by serial intravascular ultrasound analyses: data from the randomized TAXUS‐II trial. Circulation 2004109196–200. [DOI] [PubMed] [Google Scholar]
- 34.Altman D G. Confidence intervals for the number needed to treat. BMJ 19983171309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn A V, Palacios I F, Kastrati A.et al Drug‐eluting stents for diabetes mellitus: a rush to judgment? J Am Coll Cardiol 200545479–483. [DOI] [PubMed] [Google Scholar]
- 36.Corpus R A, George P B, House J A.et al Optimal glycemic control is associated with a lower rate of target vessel revascularization in treated type II diabetic patients undergoing elective percutaneous coronary intervention. J Am Coll Cardiol 2004438–14. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert J, Raboud J, Zinman B. Meta‐analysis of the effect of diabetes on restenosis rates among patients receiving coronary angioplasty stenting. Diabetes Care 200427990–994. [DOI] [PubMed] [Google Scholar]
- 38.Song F, Altman D G, Glenny A M.et al Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta‐analyses. BMJ 2003326472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morice M C. Eight‐month outcome of the REALITY study: a prospective randomized multi‐center head‐to‐head comparison of the sirolimus‐eluting stent (Cypher) and the paclitaxel‐eluting stent (Taxus) [video]. American College of Cardiology, Annual Scientific Session 2005, Orlando, 6–9 March 2005. www.acc05online.acc.org/highlights/keyLectures.aspx?sessionId = 7890&&date = 6 (accessed 8 September 2005)
- 40.Hoffmann R, Mintz G S, Dussaillant G R.et al Patterns and mechanisms of in‐stent restenosis: a serial intravascular ultrasound study. Circulation 1996941247–1254. [DOI] [PubMed] [Google Scholar]
- 41.Farb A, Heller P F, Shroff S.et al Pathological analysis of local delivery of paclitaxel via a polymer‐coated stent. Circulation 2001104473–479. [DOI] [PubMed] [Google Scholar]
- 42.Schiff P B, Horwitz S B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980771561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallo R, Padurean A, Jayaraman T.et al Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 1999992164–2170. [DOI] [PubMed] [Google Scholar]
- 44.Burke S E, Lubbers N L, Chen Y W.et al Neointimal formation after balloon‐induced vascular injury in Yucatan minipigs is reduced by oral rapamycin. J Cardiovasc Pharmacol 199933829–835. [DOI] [PubMed] [Google Scholar]
- 45.Poon M, Marx S O, Gallo R.et al Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest 1996982277–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kornowski R, Mintz G S, Lansky A J.et al Paradoxic decreases in atherosclerotic plaque mass in insulin‐treated diabetic patients. Am J Cardiol 1998811298–1304. [DOI] [PubMed] [Google Scholar]
- 47.Babinska A, Markell M S, Salifu M O.et al Enhancement of human platelet aggregation and secretion induced by rapamycin. Nephrol Dial Transplant 1998133153–3159. [DOI] [PubMed] [Google Scholar]
- 48.Levin A D, Vukmirovic N, Hwang C W.et al Specific binding to intracellular proteins determines arterial transport properties for rapamycin and paclitaxel. Proc Natl Acad Sci U S A 20041019463–9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kastrati A, Mehilli J, Dirschinger J.et al Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR‐STEREO) trial. Circulation 20011032816–2821. [DOI] [PubMed] [Google Scholar]
- 50.Pache J, Kastrati A, Mehilli J et a l. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR‐STEREO‐2) trial. J Am Coll Cardiol 2003411283–1288. [DOI] [PubMed] [Google Scholar]
- 51.Deeks J J. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Stat Med 2002211575–1600. [DOI] [PubMed] [Google Scholar]