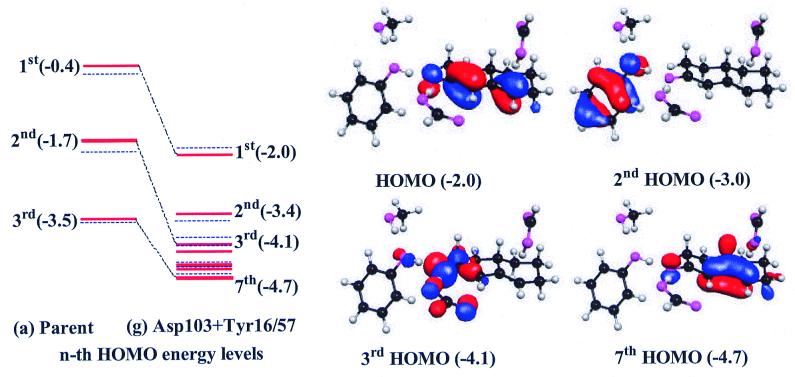
Figure 3.
Comparison of TS2 HOMOs of two model systems, (a) Parent and (g) Asp-103 + Tyr-16/57. The MO energy levels (in eV) are drawn in red and blue lines for ɛ = 1 and 10, respectively. The case for ɛ = 80 (not drawn) is similar to that for ɛ = 10. In case a, the HOMO energy of TS2 is high (−0.4 eV) compared with that of ES (−1.7 eV), because a negative charge needs to be stored in the substrate. However, the HOMO energy of ES through EP in case g is somewhat constant and highly negative (−2.0 to ≈−2.7 eV). Thus, the MOs clearly demonstrate how the catalytic residues of case g lower the activation barrier by the proton–electron rearrangements driven by SSHB compared with case a. For g, a strong π-conjugation is responsible for the change in bond orders (electron rearrangements). This π-conjugation lowers the HOMO energy drastically (−2.0 eV), because the negative charge in the substrate responsible for raising the HOMO energy of a is temporarily stored on catalytic residues Tyr-16 and Asp-103 at second to sixth HOMOs (−3.4, −4.1, −4.4, −4.6, and −4.6 eV). The seventh MO energy showing the full π-conjugation through four C atoms 3–6 (i.e., the same bond orders of 1.5 for all these carbon—carbon conjugate bonds), which is responsible for the H shift from C4 to C6 position, is also low (−4.7 eV). This low energy is in contrast to the high energy (−3.5 eV) of the corresponding MO for the full π-conjugation in case a. In the third HOMO of g, which corresponds to the second HOMO of a, the oxyanion is highly stabilized by its interaction with the H atoms of residues Tyr-16 and Asp-103. These H atoms are highly deshielded (and are therefore responsible for large chemical shifts) by two strongly electron-withdrawing O atoms of the residues, whereas each deshielded H atom (or proton) shared by both anionic O atoms shows some of the highly polarized p-like orbital characteristics (due to the sp hybridization with which the proton bridges the two O atoms). This analysis in a way reflects the characteristics of SSHBs due to MO interactions as well as noninduced electrostatic interactions.