Abstract
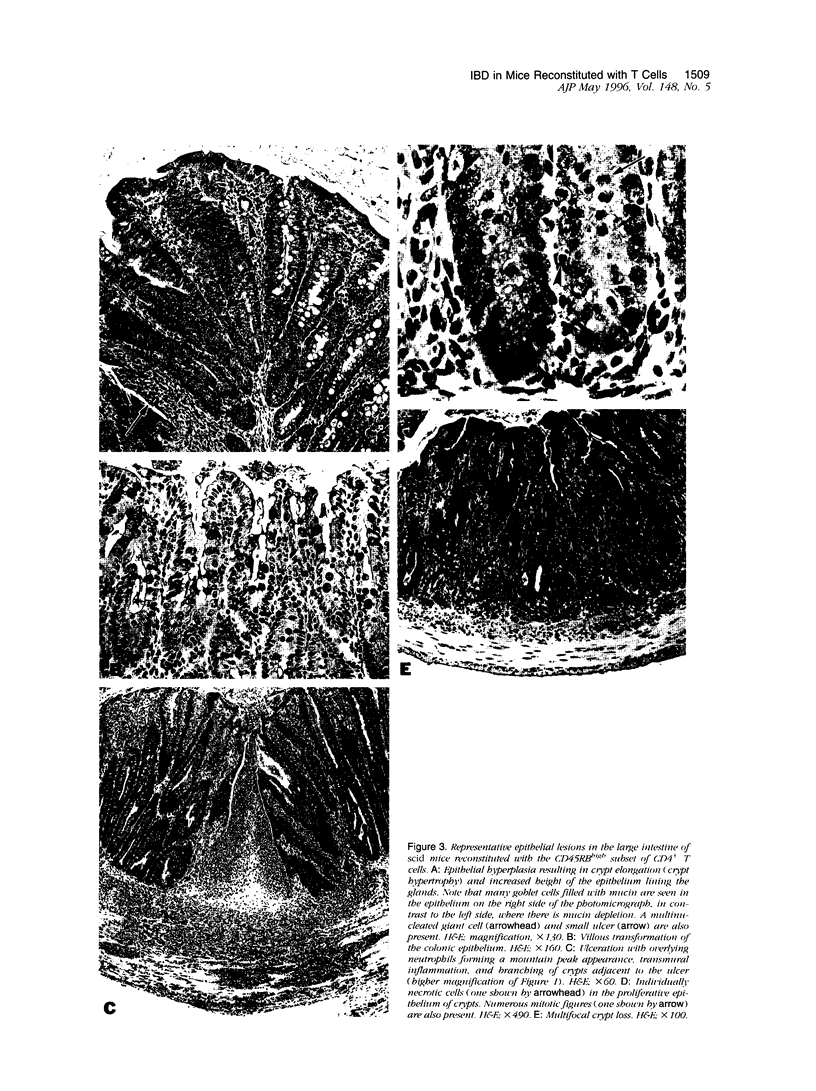
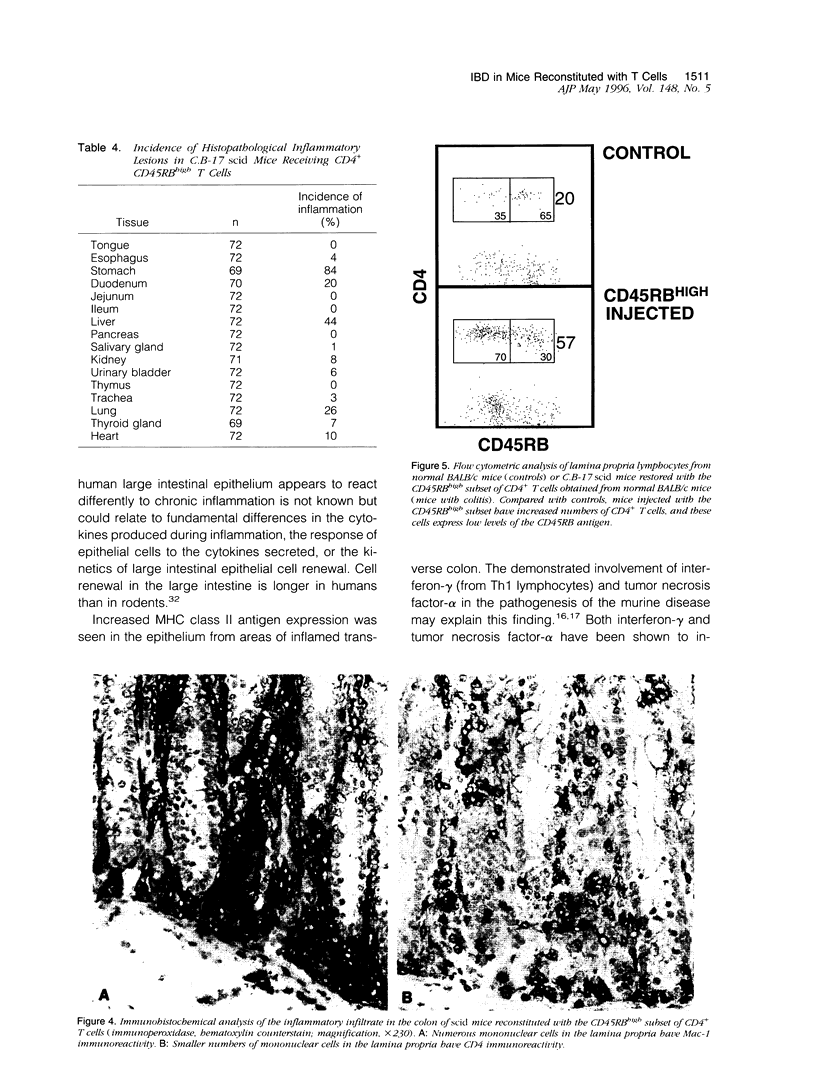
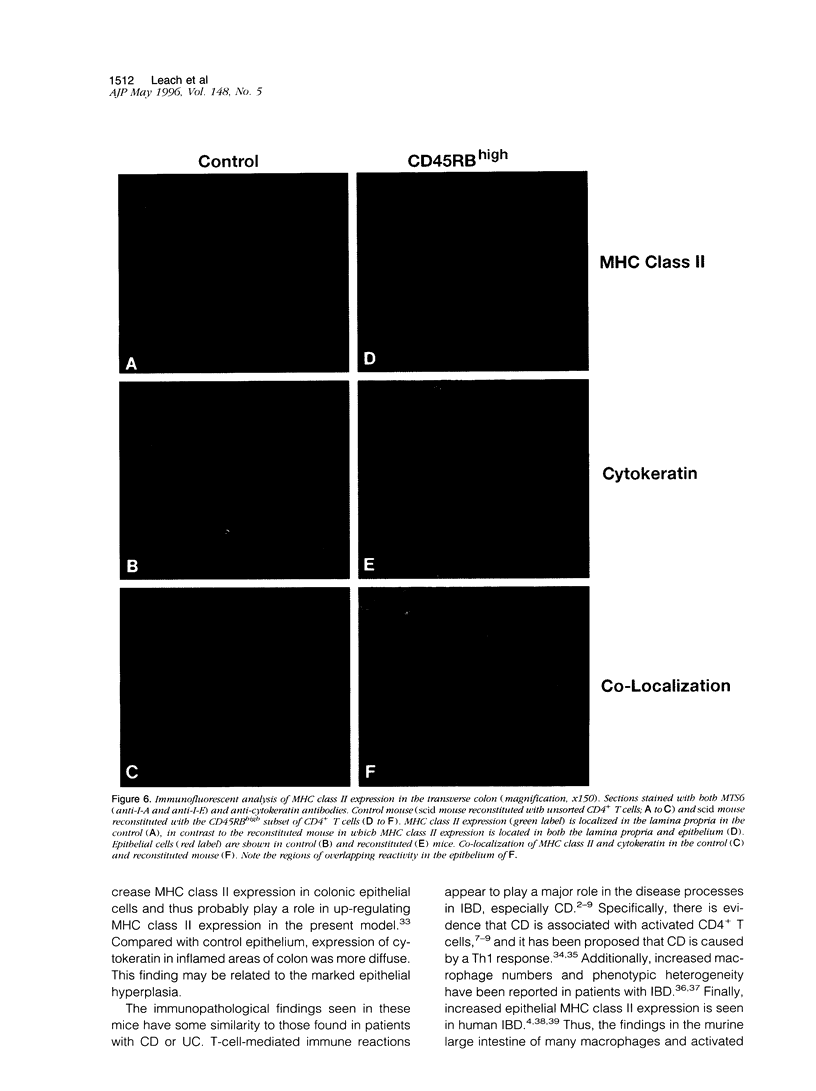
Chronic inflammation developed spontaneously in the large intestine of C.B-17 scid mice restored with the CD45RBhigh subset of CD4+ T cells obtained from normal BALB/c mice. The inflammation, which extended diffusely from the cecum to the rectum, was localized to the lamina propria of mildly affected mice but became transmural in severely affected mice. Immunohistochemical and flow cytometric analyses showed that the inflammatory infiltrate contained numerous macrophages accompanied by moderate numbers of activated CD4+ lymphocytes. Some mice also had scattered multinucleated giant cells. Mucin depletion and epithelial hyperplasia resulting in glandular elongation and mucosal thickening were also consistently seen. Less frequent findings included ulceration with fibrosis, crypt abscesses, crypt loss, and granulomatous inflammation. Immunofluorescent analysis of inflamed large intestinal sections demonstrated increased epithelial expression of major histocompatibility class II antigens. The changes in the large intestine of these mice are similar to those seen in patients with idiopathic inflammatory bowel disease (Crohn's disease and ulcerative colitis). This murine model may be useful for studying mucosal immunoregulation as it relates to the pathogenesis and treatment of chronic inflammatory bowel diseases in the large intestine of human patients.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg J., Ebert E. C., Mayer L. T-cell activation in human intestinal mucosa: the role of superantigens. Gastroenterology. 1993 Nov;105(5):1421–1430. doi: 10.1016/0016-5085(93)90147-5. [DOI] [PubMed] [Google Scholar]
- Allison M. C., Cornwall S., Poulter L. W., Dhillon A. P., Pounder R. E. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988 Nov;29(11):1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autschbach F., Schürmann G., Qiao L., Merz H., Wallich R., Meuer S. C. Cytokine messenger RNA expression and proliferation status of intestinal mononuclear cells in noninflamed gut and Crohn's disease. Virchows Arch. 1995;426(1):51–60. doi: 10.1007/BF00194698. [DOI] [PubMed] [Google Scholar]
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin G. P., O'Toole B. A., Orsmby I. E., Diebold R. J., Eis M. J., Doetschman T., Kier A. B. Onset and progression of pathological lesions in transforming growth factor-beta 1-deficient mice. Am J Pathol. 1995 Jan;146(1):276–288. [PMC free article] [PubMed] [Google Scholar]
- Bottomly K., Luqman M., Greenbaum L., Carding S., West J., Pasqualini T., Murphy D. B. A monoclonal antibody to murine CD45R distinguishes CD4 T cell populations that produce different cytokines. Eur J Immunol. 1989 Apr;19(4):617–623. doi: 10.1002/eji.1830190407. [DOI] [PubMed] [Google Scholar]
- Braegger C. P., MacDonald T. T. Immune mechanisms in chronic inflammatory bowel disease. Ann Allergy. 1994 Feb;72(2):135–141. [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Bucy R. P., Xu X. Y., Li J., Huang G. Cyclosporin A-induced autoimmune disease in mice. J Immunol. 1993 Jul 15;151(2):1039–1050. [PubMed] [Google Scholar]
- Choy M. Y., Walker-Smith J. A., Williams C. B., MacDonald T. T. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. S., Murthy S. N., Shah R. S., Sedergran D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993 Aug;69(2):238–249. [PubMed] [Google Scholar]
- Eastwood G. L. Gastrointestinal epithelial renewal. Gastroenterology. 1977 May;72(5 Pt 1):962–975. [PubMed] [Google Scholar]
- Eigenbrodt M. L., Eigenbrodt E. H., Thiele D. L. Histologic similarity of murine colonic graft-versus-host disease (GVHD) to human colonic GVHD and inflammatory bowel disease. Am J Pathol. 1990 Nov;137(5):1065–1076. [PMC free article] [PubMed] [Google Scholar]
- Evans C. M., Phillips A. D., Walker-Smith J. A., MacDonald T. T. Activation of lamina propria T cells induces crypt epithelial proliferation and goblet cell depletion in cultured human fetal colon. Gut. 1992 Feb;33(2):230–235. doi: 10.1136/gut.33.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin W. A., McDonald G. B., Stein H. O., Gatter K. C., Jewell D. P., Clarke L. C., Mason D. Y. Immunohistologic demonstration of abnormal colonic crypt cell kinetics in ulcerative colitis. Hum Pathol. 1985 Nov;16(11):1129–1132. doi: 10.1016/s0046-8177(85)80181-7. [DOI] [PubMed] [Google Scholar]
- Hibi T., Ohara M., Toda K., Hara A., Ogata H., Iwao Y., Watanabe N., Watanabe M., Hamada Y., Kobayashi K. In vitro anticolon antibody production by mucosal or peripheral blood lymphocytes from patients with ulcerative colitis. Gut. 1990 Dec;31(12):1371–1376. doi: 10.1136/gut.31.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ishii N., Chiba M., Iizuka M., Horie Y., Masamune O. Induction of HLA-DR antigen expression on human colonic epithelium by tumor necrosis factor-alpha and interferon-gamma. Scand J Gastroenterol. 1994 Oct;29(10):903–907. doi: 10.3109/00365529409094861. [DOI] [PubMed] [Google Scholar]
- Karttunnen R., Breese E. J., Walker-Smith J. A., MacDonald T. T. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994 Nov;47(11):1015–1018. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast D. A simple index for the measurement of the runting syndrome and its use in the study of the influence of the gut flora in its production. Immunology. 1968 Aug;15(2):237–245. [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Ward J. M., Yaswen L., Mackall C. L., Bauer S. R., Huh C. G., Gress R. E., Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995 Jan;146(1):264–275. [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. Gastrointestinal inflammation. Inflammatory bowel disease in knockout mice. Curr Biol. 1994 Mar 1;4(3):261–263. doi: 10.1016/s0960-9822(00)00060-9. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. The role of activated T lymphocytes in gastrointestinal disease. Clin Exp Allergy. 1990 May;20(3):247–252. doi: 10.1111/j.1365-2222.1990.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Meuwissen S. G., Feltkamp-Vroom T. M., De La Rivière A. B., Von Dem Borne A. E., Tytgat G. N. Analysis of the lympho-plasmacytic infiltrate in Crohn's disease with special reference to identification of lymphocyte-subpopulations. Gut. 1976 Oct;17(10):770–780. doi: 10.1136/gut.17.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell I. C., Turk J. L. An experimental animal model of granulomatous bowel disease. Gut. 1989 Oct;30(10):1371–1378. doi: 10.1136/gut.30.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Grusby M. J., Glimcher L. H., Bhan A. K., Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K., Braddy S., Liggitt D., Watson J. D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993 Jul 1;178(1):237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher P., Nielsen S. T., Rainsford K. D. Inflammatory bowel disease induced by combined bacterial immunization and oral carrageenan in guinea pigs. Model development, histopathology, and effects of sulfasalazine. Dig Dis Sci. 1991 Apr;36(4):461–470. doi: 10.1007/BF01298875. [DOI] [PubMed] [Google Scholar]
- Pirzer U., Schönhaar A., Fleischer B., Hermann E., Meyer zum Büschenfelde K. H. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn's disease. Lancet. 1991 Nov 16;338(8777):1238–1239. doi: 10.1016/0140-6736(91)92104-a. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Schmelkin I., Burton D. A., August A., McGrath H., Mayer L. F. T cell antigen receptor V gene usage. Increases in V beta 8+ T cells in Crohn's disease. J Clin Invest. 1990 Jun;85(6):1770–1776. doi: 10.1172/JCI114634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Caddle L. B., Coffman R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Rosekrans P. C., Meijer C. J., van der Wal A. M., Cornelisse C. J., Lindeman J. Immunoglobulin containing cells in inflammatory bowel disease of the colon: a morphometric and immunohistochemical study. Gut. 1980 Nov;21(11):941–947. doi: 10.1136/gut.21.11.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugtveit J., Brandtzaeg P., Halstensen T. S., Fausa O., Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994 May;35(5):669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Storb R., Prentice R. L., Buckner C. D., Clift R. A., Appelbaum F., Deeg J., Doney K., Hansen J. A., Mason M., Sanders J. E. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983 Feb 10;308(6):302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Riddell R. H. The pathological diagnosis and differential diagnosis of Crohn's disease. Hepatogastroenterology. 1990 Feb;37(1):18–31. [PubMed] [Google Scholar]
- Targan S. R., Deem R. L., Shanahan F. Role of mucosal T-cell-generated cytokines in epithelial cell injury. Immunol Res. 1991;10(3-4):472–478. doi: 10.1007/BF02919744. [DOI] [PubMed] [Google Scholar]
- Thayer W. R., Jr, Brown M., Sangree M. H., Katz J., Hersh T. Escherichia Coli O:14 and colon hemagglutinating antibodies in inflammatory bowel disease. Gastroenterology. 1969 Sep;57(3):311–318. [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Badr-el-Din S., Smart C. J., Malizia G., Oakes D. J., Heatley R. V., Losowsky M. S. Colonic mucosal T lymphocytes in ulcerative colitis: expression of CD7 antigen in relation to MHC class II (HLA-D) antigens. Dig Dis Sci. 1989 Sep;34(9):1449–1456. doi: 10.1007/BF01538084. [DOI] [PubMed] [Google Scholar]
- van Bekkum D. W., Roodenburg J., Heidt P. J., van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974 Feb;52(2):401–404. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]