Abstract
The function of apolipoprotein J (apoJ) is unknown, but it has been hypothesized to be cytoprotective. In the normal heart, abundant apoJ mRNA and protein are expressed in atrial myocytes; no expression is detected in ventricular myocytes. To provide clues about the role of apoJ in the heart, the response of apoJ to heart disease, including three models of myocarditis and two models of in vivo pressure overload hypertrophy, were examined. In the disease model studied extensively, myosin-induced myocarditis, in situ hybridization detected induction of apoJ mRNA in ventricular myocytes immediately before histological evidence of injury. ApoJ message in ventricular myocytes reached high levels as myocarditis became more severe. Evidence of early apoJ induction, before inflammation and injury, also occurred in viral myocarditis. ApoJ mRNA was not present in the inflammatory or interstitial cells during myocarditis. In areas of severe inflammation and myocardial fiber degeneration, apoJ showed a gradient of expression, with highest levels in myocytes immediately surrounding the lesion and diminishing with increasing distance. ApoJ protein also accumulated in myocytes at the interface between degenerated myocardial tissue and the surrounding cardiac tissue. During cardiac hypertrophy that occurred without associated inflammation or cell damage, ventricular apoJ mRNA was not detected. When ischemic damage accompanied hypertrophy, apoJ was induced in the ventricular myocytes near the lesion borders. The correlation of apoJ induction with ventricular tissue damage, but not hypertrophy, suggests that apoJ is a repair response protein. We propose that apoJ functions to limit tissue injury and/or promote tissue remodeling.
Full text
PDF



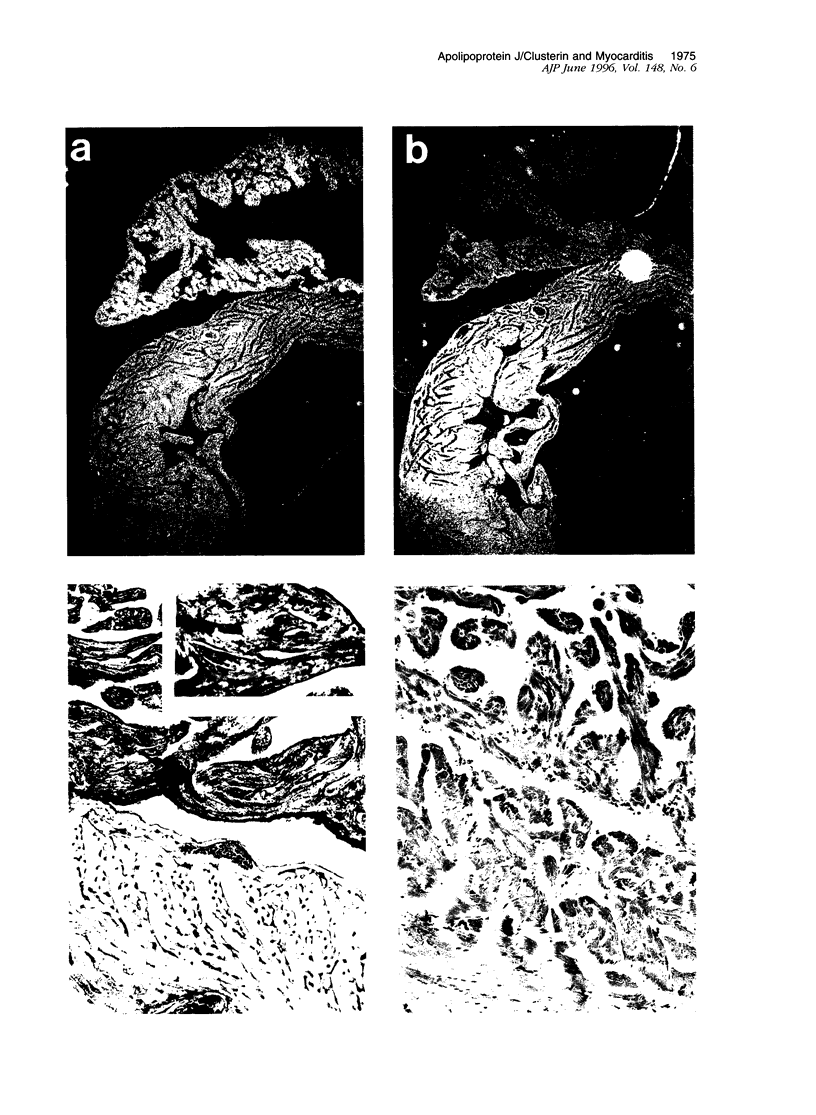








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B. J., Lund S. D., Brown T. L., Harmony J. A., Witte D. P. Apolipoprotein J expression at fluid-tissue interfaces: potential role in barrier cytoprotection. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):725–729. doi: 10.1073/pnas.90.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S. P., Melsen L. R. Myocardial necrosis, fibrosis, and DNA synthesis in experimental cardiac hypertrophy induced by sudden pressure overload. Circ Res. 1976 Aug;39(2):238–245. doi: 10.1161/01.res.39.2.238. [DOI] [PubMed] [Google Scholar]
- Brown T. L., Moulton B. C., Baker V. V., Mira J., Harmony J. A. Expression of apolipoprotein J in the uterus is associated with tissue remodeling. Biol Reprod. 1995 May;52(5):1038–1049. doi: 10.1095/biolreprod52.5.1038. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Miura N. H., Ihara Y., Fukuchi K., Takeda M., Nakano Y., Tobe T., Tomita M. SP-40,40 is a constituent of Alzheimer's amyloid. Acta Neuropathol. 1992;83(3):260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Correa-Rotter R., Hostetter T. H., Manivel J. C., Eddy A. A., Rosenberg M. E. Intrarenal distribution of clusterin following reduction of renal mass. Kidney Int. 1992 Apr;41(4):938–950. doi: 10.1038/ki.1992.144. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Frank M. M. Complement in the pathophysiology of human disease. N Engl J Med. 1987 Jun 11;316(24):1525–1530. doi: 10.1056/NEJM198706113162407. [DOI] [PubMed] [Google Scholar]
- Guenette R. S., Corbeil H. B., Léger J., Wong K., Mézl V., Mooibroek M., Tenniswood M. Induction of gene expression during involution of the lactating mammary gland of the rat. J Mol Endocrinol. 1994 Feb;12(1):47–60. doi: 10.1677/jme.0.0120047. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis. Identification of different pathogenic mechanisms in DBA/2 and Balb/c mice. Am J Pathol. 1986 Feb;122(2):284–291. [PMC free article] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Jordan-Starck T. C., Lund S. D., Witte D. P., Aronow B. J., Ley C. A., Stuart W. D., Swertfeger D. K., Clayton L. R., Sells S. F., Paigen B. Mouse apolipoprotein J: characterization of a gene implicated in atherosclerosis. J Lipid Res. 1994 Feb;35(2):194–210. [PubMed] [Google Scholar]
- Kounnas M. Z., Loukinova E. B., Stefansson S., Harmony J. A., Brewer B. H., Strickland D. K., Argraves W. S. Identification of glycoprotein 330 as an endocytic receptor for apolipoprotein J/clusterin. J Biol Chem. 1995 Jun 2;270(22):13070–13075. doi: 10.1074/jbc.270.22.13070. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampert-Etchells M., McNeill T. H., Laping N. J., Zarow C., Finch C. E., May P. C. Sulfated glycoprotein-2 is increased in rat hippocampus following entorhinal cortex lesioning. Brain Res. 1991 Nov 1;563(1-2):101–106. doi: 10.1016/0006-8993(91)91520-b. [DOI] [PubMed] [Google Scholar]
- Lieberman E. B., Hutchins G. M., Herskowitz A., Rose N. R., Baughman K. L. Clinicopathologic description of myocarditis. J Am Coll Cardiol. 1991 Dec;18(7):1617–1626. doi: 10.1016/0735-1097(91)90493-s. [DOI] [PubMed] [Google Scholar]
- May P. C., Finch C. E. Sulfated glycoprotein 2: new relationships of this multifunctional protein to neurodegeneration. Trends Neurosci. 1992 Oct;15(10):391–396. doi: 10.1016/0166-2236(92)90190-j. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Kawamata T., Walker D. G. Distribution of clusterin in Alzheimer brain tissue. Brain Res. 1992 May 8;579(2):337–341. doi: 10.1016/0006-8993(92)90071-g. [DOI] [PubMed] [Google Scholar]
- McRitchie D. I., Girotti M. J., Glynn M. F., Goldberg J. M., Rotstein O. D. Effect of systemic fibrinogen depletion on intraabdominal abscess formation. J Lab Clin Med. 1991 Jul;118(1):48–55. [PubMed] [Google Scholar]
- Moestrup S. K., Nielsen S., Andreasen P., Jørgensen K. E., Nykjaer A., Røigaard H., Gliemann J., Christensen E. I. Epithelial glycoprotein-330 mediates endocytosis of plasminogen activator-plasminogen activator inhibitor type-1 complexes. J Biol Chem. 1993 Aug 5;268(22):16564–16570. [PubMed] [Google Scholar]
- Nath K. A., Dvergsten J., Correa-Rotter R., Hostetter T. H., Manivel J. C., Rosenberg M. E. Induction of clusterin in acute and chronic oxidative renal disease in the rat and its dissociation from cell injury. Lab Invest. 1994 Aug;71(2):209–218. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Pasinetti G. M., Finch C. E. Sulfated glycoprotein-2 (SGP-2) mRNA is expressed in rat striatal astrocytes following ibotenic acid lesions. Neurosci Lett. 1991 Sep 2;130(1):1–4. doi: 10.1016/0304-3940(91)90213-d. [DOI] [PubMed] [Google Scholar]
- Rockman H. A., Ono S., Ross R. S., Jones L. R., Karimi M., Bhargava V., Ross J., Jr, Chien K. R. Molecular and physiological alterations in murine ventricular dysfunction. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2694–2698. doi: 10.1073/pnas.91.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman H. A., Ross R. S., Harris A. N., Knowlton K. U., Steinhelper M. E., Field L. J., Ross J., Jr, Chien K. R. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman H. A., Wachhorst S. P., Mao L., Ross J., Jr ANG II receptor blockade prevents ventricular hypertrophy and ANF gene expression with pressure overload in mice. Am J Physiol. 1994 Jun;266(6 Pt 2):H2468–H2475. doi: 10.1152/ajpheart.1994.266.6.H2468. [DOI] [PubMed] [Google Scholar]
- Rose N. R., Neumann D. A., Herskowitz A., Traystman M. D., Beisel K. W. Genetics of susceptibility to viral myocarditis in mice. Pathol Immunopathol Res. 1988;7(4):266–278. doi: 10.1159/000157122. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Paller M. S. Differential gene expression in the recovery from ischemic renal injury. Kidney Int. 1991 Jun;39(6):1156–1161. doi: 10.1038/ki.1991.146. [DOI] [PubMed] [Google Scholar]
- Rouleau M., Léger J., Tenniswood M. Ductal heterogeneity of cytokeratins, gene expression, and cell death in the rat ventral prostate. Mol Endocrinol. 1990 Dec;4(12):2003–2013. doi: 10.1210/mend-4-12-2003. [DOI] [PubMed] [Google Scholar]
- Rozovsky I., Morgan T. E., Willoughby D. A., Dugichi-Djordjevich M. M., Pasinetti G. M., Johnson S. A., Finch C. E. Selective expression of clusterin (SGP-2) and complement C1qB and C4 during responses to neurotoxins in vivo and in vitro. Neuroscience. 1994 Oct;62(3):741–758. doi: 10.1016/0306-4522(94)90473-1. [DOI] [PubMed] [Google Scholar]
- Saito A., Pietromonaco S., Loo A. K., Farquhar M. G. Complete cloning and sequencing of rat gp330/"megalin," a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Aminian A., McRae J. L., O'Farrell K. A., Adam W. R., Murphy B. F. Clusterin depletion enhances immune glomerular injury in the isolated perfused kidney. Kidney Int. 1994 Mar;45(3):817–827. doi: 10.1038/ki.1994.108. [DOI] [PubMed] [Google Scholar]
- Sawczuk I. S., Hoke G., Olsson C. A., Connor J., Buttyan R. Gene expression in response to acute unilateral ureteral obstruction. Kidney Int. 1989 Jun;35(6):1315–1319. doi: 10.1038/ki.1989.128. [DOI] [PubMed] [Google Scholar]
- Shiverick K. T., Thomas L. L., Alpert N. R. Purification of cardiac myosin. Application to hypertrophied myocardium. Biochim Biophys Acta. 1975 May 30;393(1):124–133. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A., Jones W. K., Gulick J., Wert S., Neumann J., Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991 Dec 25;266(36):24613–24620. [PubMed] [Google Scholar]
- Sylvester S. R., Skinner M. K., Griswold M. D. A sulfated glycoprotein synthesized by Sertoli cells and by epididymal cells is a component of the sperm membrane. Biol Reprod. 1984 Dec;31(5):1087–1101. doi: 10.1095/biolreprod31.5.1087. [DOI] [PubMed] [Google Scholar]
- Taketo M., Schroeder A. C., Mobraaten L. E., Gunning K. B., Hanten G., Fox R. R., Roderick T. H., Stewart C. L., Lilly F., Hansen C. T. FVB/N: an inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traystman M. D., Chow L. H., McManus B. M., Herskowitz A., Nesbitt M. N., Beisel K. W. Susceptibility to Coxsackievirus B3-induced chronic myocarditis maps near the murine Tcr alpha and Myhc alpha loci on chromosome 14. Am J Pathol. 1991 Mar;138(3):721–726. [PMC free article] [PubMed] [Google Scholar]
- Väkevä A., Laurila P., Meri S. Co-deposition of clusterin with the complement membrane attack complex in myocardial infarction. Immunology. 1993 Oct;80(2):177–182. [PMC free article] [PubMed] [Google Scholar]
- Willnow T. E., Goldstein J. L., Orth K., Brown M. S., Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem. 1992 Dec 25;267(36):26172–26180. [PubMed] [Google Scholar]
- Witte D. P., Aronow B. J., Dry J. K., Harmony J. A. Temporally and spatially restricted expression of apolipoprotein J in the developing heart defines discrete stages of valve morphogenesis. Dev Dyn. 1994 Nov;201(3):290–296. doi: 10.1002/aja.1002010311. [DOI] [PubMed] [Google Scholar]
- Witte D. P., Wiginton D. A., Hutton J. J., Aronow B. J. Coordinate developmental regulation of purine catabolic enzyme expression in gastrointestinal and postimplantation reproductive tracts. J Cell Biol. 1991 Oct;115(1):179–190. doi: 10.1083/jcb.115.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E., Brosnan C. F., Raine C. S. SP-40,40 immunoreactivity in inflammatory CNS lesions displaying astrocyte/oligodendrocyte interactions. J Neuropathol Exp Neurol. 1993 Mar;52(2):129–134. doi: 10.1097/00005072-199303000-00005. [DOI] [PubMed] [Google Scholar]
- ZINSSER H. H., PRYDE A. W. Experimental study of physical factors, including fibrin formation, influencing the spread of fluids and small particles within and from the peritoneal cavity of the dog. Ann Surg. 1952 Nov;136(5):818–827. doi: 10.1097/00000658-195211000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin R. A., Condra J. H., Dixon R. A., Seidah N. G., Chrétien M., Nemer M., Chamberland M., Drouin J. Molecular cloning and characterization of DNA sequences encoding rat and human atrial natriuretic factors. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6325–6329. doi: 10.1073/pnas.81.20.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bilsen M., Chien K. R. Growth and hypertrophy of the heart: towards an understanding of cardiac specific and inducible gene expression. Cardiovasc Res. 1993 Jul;27(7):1140–1149. doi: 10.1093/cvr/27.7.1140. [DOI] [PubMed] [Google Scholar]







