Abstract
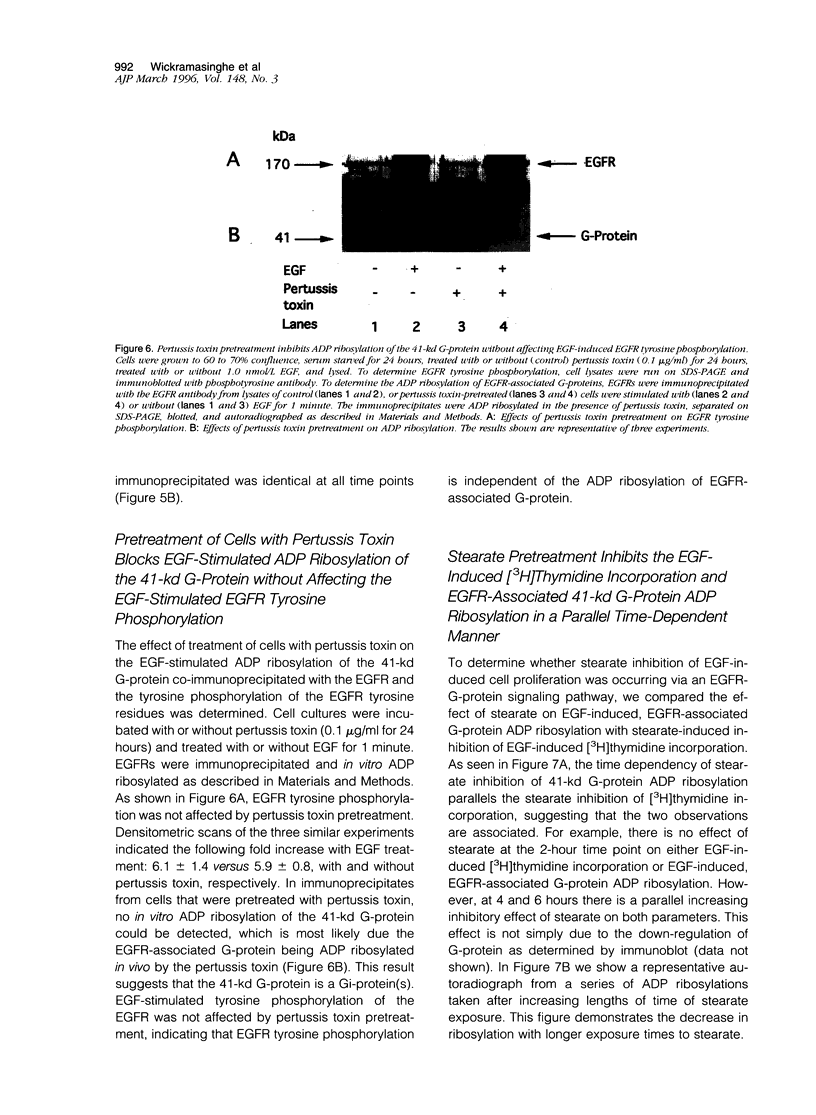
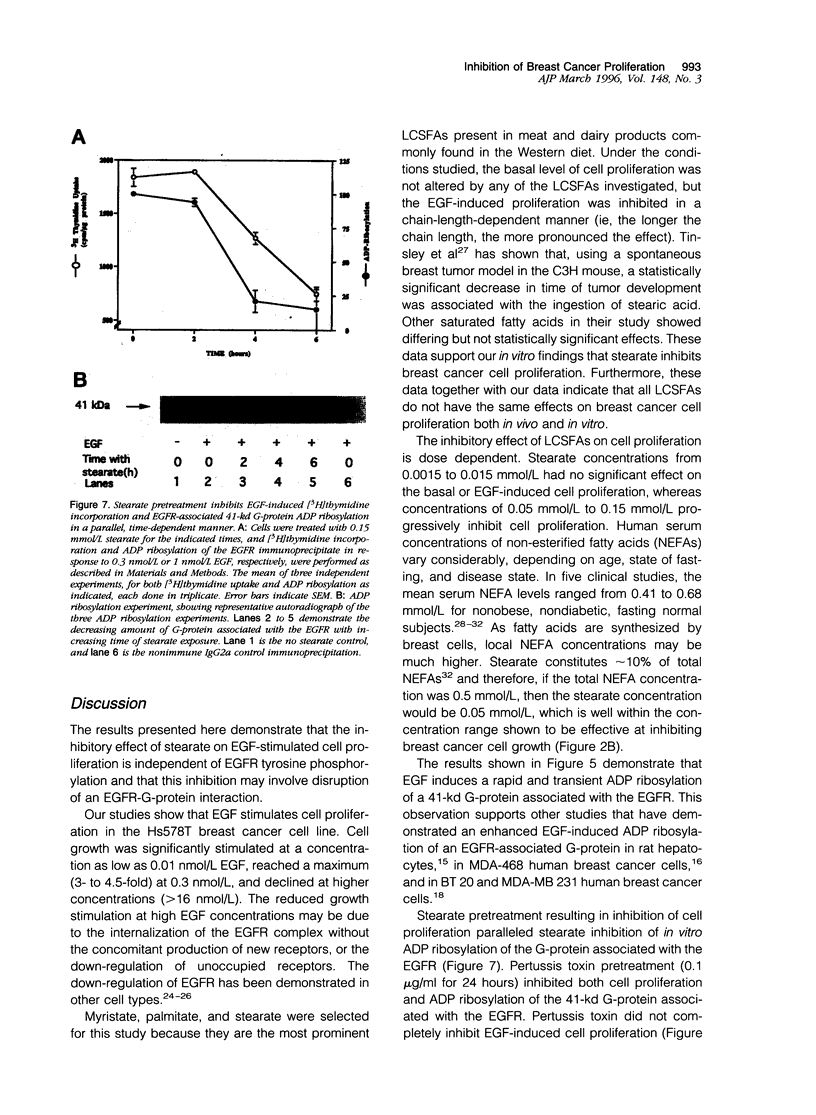
Long chain saturated fatty acids are known to inhibit breast cancer cell proliferation; however, the mechanism of this inhibition is not known. Treatment of Hs578T breast cancer cells with long chain saturated fatty acids (0.15 mmol/L for 6 hours) before epidermal growth factor (EGF) treatment inhibited EGF-induced cell proliferation in a chain-length-dependent manner. Stearate (C:18) completely inhibited the EGF-induced cell proliferation, whereas palmitate (C:16) inhibited by 67 +/- 8% and myristate (C:14) had no effect. In contrast, stearate had little effect on insulin-like growth factor-1-stimulated cell proliferation. The inhibitory effect of stearate on cell proliferation was dose and time dependent and independent of EGF receptor (EGFR) tyrosine phosphorylation. Pretreatment of cells with pertussis toxin (0.1 microgram/ml for 24 hours) inhibited the EGF-induced cell growth by 50 +/- 8%, also independent of EGFR tyrosine phosphorylation. A pertussis-toxin-sensitive, 41-kd G-protein was specifically co-immunoprecipitated with the EGFR. Pretreatment of cells with 0.15 mmol/L stearate from 0 to 6 hours inhibits, in parallel, both the EGF-induced cell proliferation and pertussis-toxin-catalyzed ADP ribosylation of the G-protein associated with the EGFR. These studies suggest that long chain saturated fatty acids inhibit EGF-induced breast cancer cell growth via a mechanism involving an EGFR-G-protein signaling pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- BIERMAN E. L., DOLE V. P., ROBERTS T. N. An abnormality of nonesterified fatty acid metabolism in diabetes mellitus. Diabetes. 1957 Nov-Dec;6(6):475–479. doi: 10.2337/diab.6.6.475. [DOI] [PubMed] [Google Scholar]
- Braden L. M., Carroll K. K. Dietary polyunsaturated fat in relation to mammary carcinogenesis in rats. Lipids. 1986 Apr;21(4):285–288. doi: 10.1007/BF02536414. [DOI] [PubMed] [Google Scholar]
- Buckman D. K., Chapkin R. S., Erickson K. L. Modulation of mouse mammary tumor growth and linoleate enhanced metastasis by oleate. J Nutr. 1990 Feb;120(2):148–157. doi: 10.1093/jn/120.2.148. [DOI] [PubMed] [Google Scholar]
- Buckman D. K., Erickson K. L. Relationship of the uptake and beta-oxidation of 18-carbon fatty acids with stimulation of murine mammary tumor cell growth. Cancer Lett. 1991 Sep;59(3):257–265. doi: 10.1016/0304-3835(91)90150-g. [DOI] [PubMed] [Google Scholar]
- Buckman D. K., Hubbard N. E., Erickson K. L. Eicosanoids and linoleate-enhanced growth of mouse mammary tumor cells. Prostaglandins Leukot Essent Fatty Acids. 1991 Nov;44(3):177–184. doi: 10.1016/0952-3278(91)90053-8. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Church J. G., Buick R. N. G-protein-mediated epidermal growth factor signal transduction in a human breast cancer cell line. Evidence for two intracellular pathways distinguishable by pertussis toxin. J Biol Chem. 1988 Mar 25;263(9):4242–4246. [PubMed] [Google Scholar]
- Crouch M. F. Growth factor-induced cell division is paralleled by translocation of Gi alpha to the nucleus. FASEB J. 1991 Feb;5(2):200–206. doi: 10.1096/fasebj.5.2.1900794. [DOI] [PubMed] [Google Scholar]
- Fraze E., Donner C. C., Swislocki A. L., Chiou Y. A., Chen Y. D., Reaven G. M. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985 Nov;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., CARLSON L. A., EKELUND L. G., HOLMGREN A. TURNOVER RATE AND OXIDATION OF DIFFERENT FREE FATTY ACIDS IN MAN DURING EXERCISE. J Appl Physiol. 1964 Jul;19:613–618. doi: 10.1152/jappl.1964.19.4.613. [DOI] [PubMed] [Google Scholar]
- Hall R. E., Lee C. S., Alexander I. E., Shine J., Clarke C. L., Sutherland R. L. Steroid hormone receptor gene expression in human breast cancer cells: inverse relationship between oestrogen and glucocorticoid receptor messenger RNA levels. Int J Cancer. 1990 Dec 15;46(6):1081–1087. doi: 10.1002/ijc.2910460622. [DOI] [PubMed] [Google Scholar]
- Hopkins G. J., Carroll K. K. Relationship between amount and type of dietary fat in promotion of mammary carcinogenesis induced by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1979 Apr;62(4):1009–1012. [PubMed] [Google Scholar]
- Hopkins G. J., Kennedy T. G., Carroll K. K. Polyunsaturated fatty acids as promoters of mammary carcinogenesis induced in Sprague-Dawley rats by 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1981 Mar;66(3):517–522. [PubMed] [Google Scholar]
- Klijn J. G., Berns P. M., Schmitz P. I., Foekens J. A. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992 Feb;13(1):3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macias A., Azavedo E., Hägerström T., Klintenberg C., Pérez R., Skoog L. Prognostic significance of the receptor for epidermal growth factor in human mammary carcinomas. Anticancer Res. 1987 May-Jun;7(3 Pt B):459–464. [PubMed] [Google Scholar]
- Mansour E. G., Ravdin P. M., Dressler L. Prognostic factors in early breast carcinoma. Cancer. 1994 Jul 1;74(1 Suppl):381–400. doi: 10.1002/cncr.2820741326. [DOI] [PubMed] [Google Scholar]
- Rose D. P., Connolly J. M. Effects of fatty acids and inhibitors of eicosanoid synthesis on the growth of a human breast cancer cell line in culture. Cancer Res. 1990 Nov 15;50(22):7139–7144. [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Seuwen K., Pouysségur J. G protein-controlled signal transduction pathways and the regulation of cell proliferation. Adv Cancer Res. 1992;58:75–94. doi: 10.1016/s0065-230x(08)60291-2. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Daidone M. G., Valagussa P., Di Fronzo G., Mezzanotte G., Mariani L., Bonadonna G. 3H-thymidine-labeling index as a prognostic indicator in node-positive breast cancer. J Clin Oncol. 1990 Aug;8(8):1321–1326. doi: 10.1200/JCO.1990.8.8.1321. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Hoak J. C. An improved method for the addition of long-chain free fatty acid to protein solutions. Anal Biochem. 1969 Nov;32(2):297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- Swislocki A. L., Chen Y. D., Golay A., Chang M. O., Reaven G. M. Insulin suppression of plasma-free fatty acid concentration in normal individuals and patients with type 2 (non-insulin-dependent) diabetes. Diabetologia. 1987 Aug;30(8):622–626. doi: 10.1007/BF00277318. [DOI] [PubMed] [Google Scholar]
- Tinsley I. J., Schmitz J. A., Pierce D. A. Influence of dietary fatty acids on the incidence of mammary tumors in the C3H mouse. Cancer Res. 1981 Apr;41(4):1460–1465. [PubMed] [Google Scholar]
- Vlodavsky I., Brown K. D., Gospodarowicz D. A comparison of the binding of epidermal growth factor to cultured granulosa and luteal cells. J Biol Chem. 1978 May 25;253(10):3744–3750. [PubMed] [Google Scholar]
- Welsch C. W. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992 Apr 1;52(7 Suppl):2040s–2048s. [PubMed] [Google Scholar]
- Wirth A., Neermann G., Eckert W., Heuck C. C., Weicker H. Metabolic response to heavy physical exercise before and after a 3-month training period. Eur J Appl Physiol Occup Physiol. 1979 Apr 12;41(1):51–59. doi: 10.1007/BF00424468. [DOI] [PubMed] [Google Scholar]
- Yang L. J., Baffy G., Rhee S. G., Manning D., Hansen C. A., Williamson J. R. Pertussis toxin-sensitive Gi protein involvement in epidermal growth factor-induced activation of phospholipase C-gamma in rat hepatocytes. J Biol Chem. 1991 Nov 25;266(33):22451–22458. [PubMed] [Google Scholar]