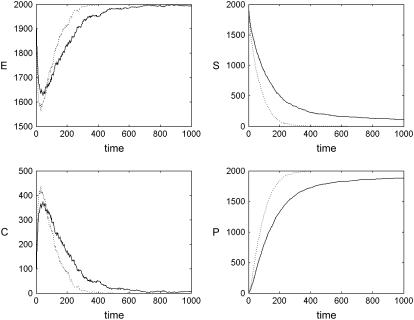
FIGURE 6.
Effect of fixed obstacles on chemical kinetics. Kinetics of the Michaelis-Menten reaction system (Eq. 5) θ = 0 (i.e., in the presence of no obstacles, dotted line) and θ = 0.4 (solid line) obstacle densities, respectively. The latter is close to the percolation threshold of θ ≈ 0.4073. When obstacles are present, the kinetics are considerably slower, especially at large times, because of the difficulty that molecules initially placed far apart have in meeting one another.