Figure 5.
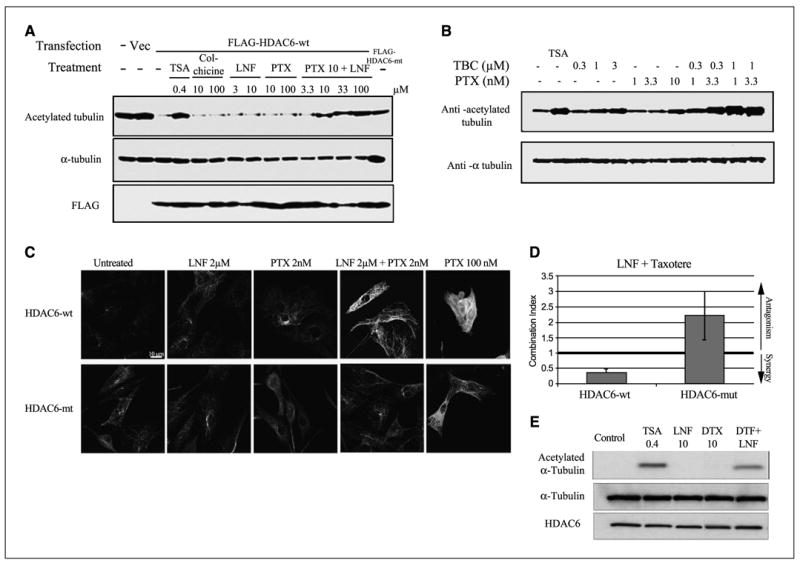
The synergistic combination of lonafarnib and taxane prevents HDAC6 tubulin deacetylation in vitro and is dependent on HDAC6 functionality. A, representative Western blots of acetylated α -tubulin, total tubulin, and Flag, following immunoprecipitation (IP) from A549 cells transfected with either Flag-HDAC6-WT or Flag-HDAC6-mut. Before Western blotting the Flag-IP complexes were incubated in vitro with preassembled purified bovine brain microtubule protein in the presence of various drugs to determine the tubulin deacetylase activity of HDAC6 (left-hand blot). B, Western blotting of acetylated tubulin after treatment with tubacin, a specific HDAC6 inhibitor, both alone and in combination with paclitaxel. Trichostatin A (TSA) was used as additional positive control for pan-HDAC inhibition. As a control for total tubulin levels, blots were reprobed for α -tubulin. C, immunofluorescence analyses of acetylated tubulin in NIH-3T3 cells stably expressing either HDAC6-wt or HDAC-mut, following 16-hour drug treatments as indicated. D, assessment of synergy between lonafarnib and docetaxel in HDAC6-wt and HDAC6-mut using combination index analysis. The lonafarnib/docetaxel combination is synergistic in HDAC6-wt (combination index < 1) but is antagonistic in HDAC6-mt cells (combination index > 1). Bars, SD. E, representative Western blots of acetylated α -tubulin, total tubulin, and HDAC6 following immunoprecipitation (IP) from NIH-3T3 cells stably expressing Flag-HDAC6-WT. Before Western blotting the Flag-IP complexes were incubated in vitro with preassembled purified bovine brain microtubule protein in the presence of various drugs to determine the tubulin deacetylase activity of HDAC6. The in vitro effects of trichostatin A (pan-HDAC inhibitor), lonafarnib, and docetaxel (DTX) on acetylated α -tubulin are shown.
