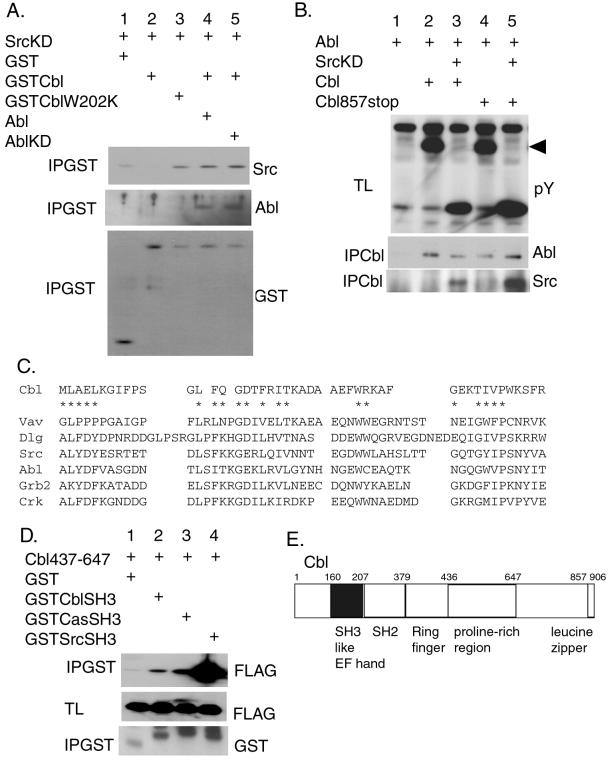
Figure 4.
The binding between SrcKD and Cbl is regulated by Abl. (A) 293T cells were transiently transfected with the indicated expression plasmids. Proteins (500 μg) from cell lysates were precipitated with glutathione beads (IPGST) and Western-blotted with the antibodies indicated at the right. (B) The monomeric Cbl mutant (Cbl857stop) was coprecipitated with Abl and Src. 293T cells were transiently transfected with the indicated plasmids. Proteins from cell lysates were immunoprecipitated by using the anti-hemagglutinin antibody Y11 to precipitate Cbl (IPCbl) and Western-blotted with the anti-Abl and anti-Src antibodies. The total lysates (TL) also were Western-blotted with anti-phosphotyrosine antibody (pY). The arrow indicates the position of Cbl. (C) Alignment of Cbl SH3-like sequence with related SH3 domains from various proteins: Vav, Drosophila disclarge protein (Dlg), Src, Abl, Grb2, and Crk. The conserved residues of the SH3 domain are indicated by *. (D) The binding of the Cbl SH3-like sequence to the proline-rich region of Cbl. Proteins (50 μg) from total lysates (TL) of the transfected 293T cells were resolved by SDS/PAGE and Western-blotted with anti-FLAG tag (FLAG) antibody recognizing the Cbl437–647 FLAG-tagged fragment. GST-fusion proteins were precipitated with glutathione beads (IPGST) and Western-blotted with the indicated antibodies. (E) The schematic representation of Cbl indicating the position of the SH3-like sequence.