Abstract
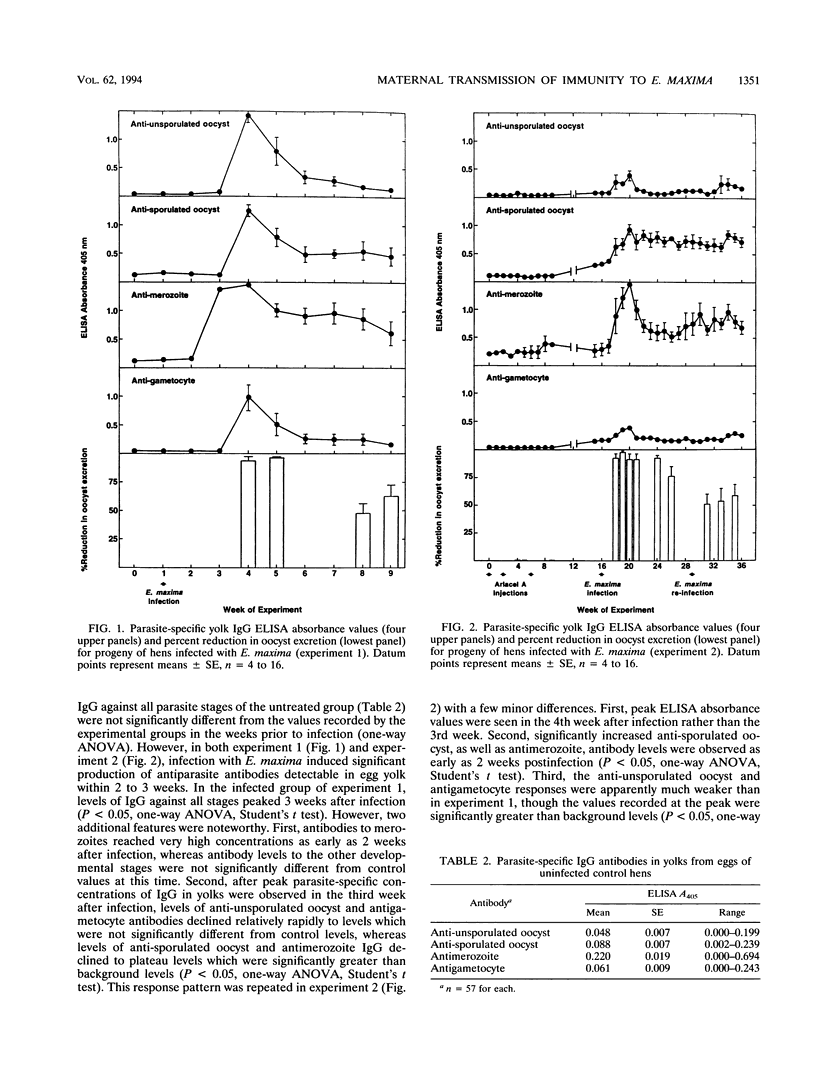
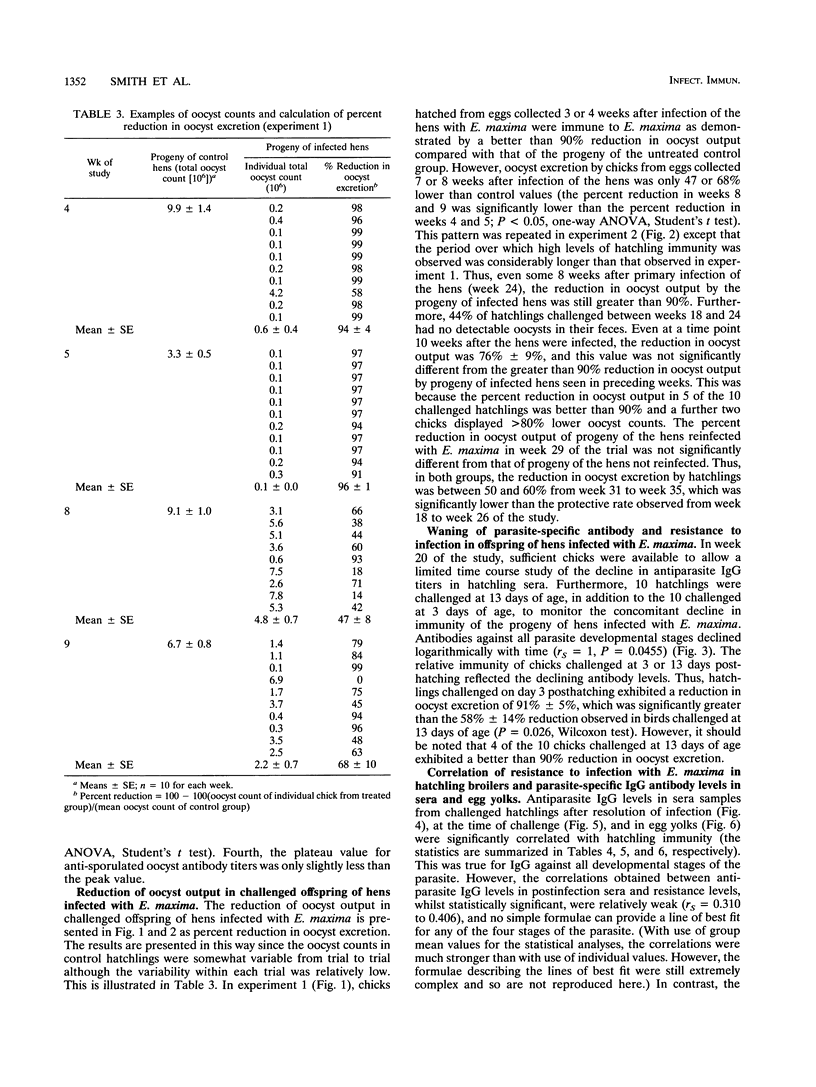
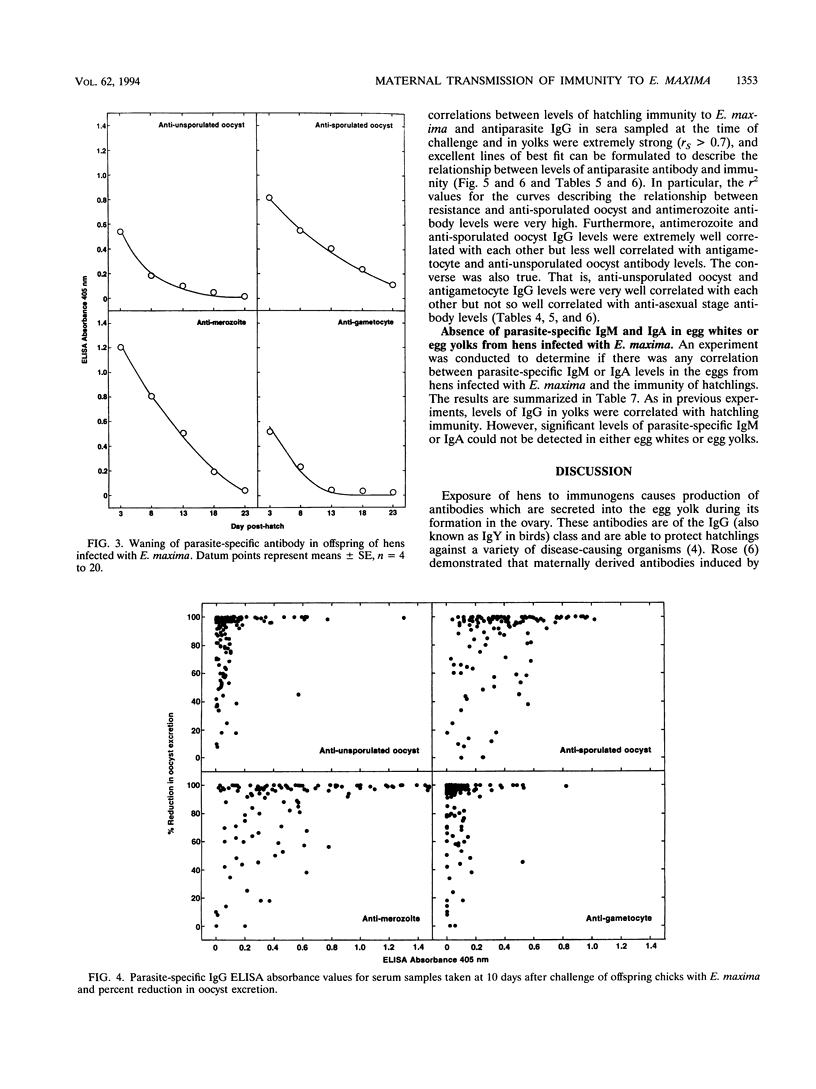
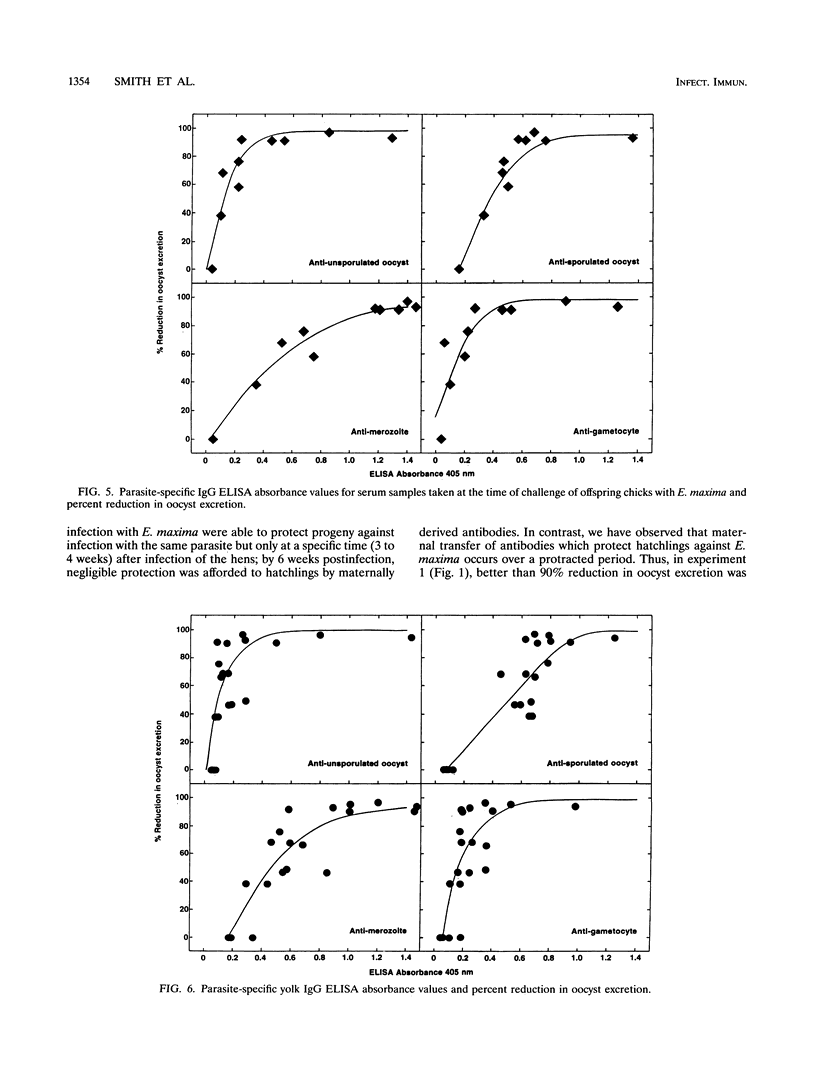
Vaccination of broiler chickens against Eimeria infection is problematic because of the need to ensure that birds are protected from the time of hatching. We have therefore investigated the feasibility of protecting hatchling broilers via maternal transfer of protective antibodies from hens to their offspring. Oral infection of broiler breeder hens with 20,000 sporulated Eimeria maxima oocysts caused production of antibodies which were passed into the egg yolk and subsequently to hatchlings. The level of specific antibodies in the yolks to unsporulated oocysts, sporulated oocysts, merozoites, and gametocytes was assessed by enzyme-linked immunosorbent assays. The levels in yolks of antibodies to all developmental stages peaked 3 to 4 weeks after infection of the hens. Groups of 10 hatchlings were challenged at 3 days of age by oral infection with 100 sporulated E. maxima oocysts. In the first experiment, the mean 4-day (days 6 to 9 post-infection) total number of oocysts excreted in the feces of chicks from eggs collected 3 weeks after infection of the hens was (0.6 +/- 0.4) x 10(6) (mean +/- standard error) compared with (9.9 +/- 1.4) x 10(6) for the progeny of uninfected hens, which represents a greater than 90% reduction. However, oocyst excretion by chicks from eggs collected 7 or 8 weeks after infection of the hens was only 47 or 68% lower than control values, reflecting declining levels of protective antibodies. In a second experiment, in which the hens were somewhat older and pretreated by intramuscular injection of saline in the emulsifying agent, Arlacel A, the period for which protective antibodies were transferred to hatchlings was prolonged. Thus, oocyst excretion by challenged hatchlings from eggs collected for an 8-week period after infection of the hens was more than 90% lower than oocyst excretion by control chicks, and even hatchlings of eggs collected 19 weeks after infection of the hens showed a 60% reduction in oocyst output. In both experiments, the levels of immunoglobulin G (IgG) antibodies to all developmental stages in yolks or hatchling sera were very strongly correlated with maternally derived immunity to E. maxima. In contrast, parasite-specific IgM or IgA was not detectable, either in egg yolk or egg white. These results demonstrate the ability of IgG antibodies to protect against E. maxima in poultry, thus raising the possibility of using protective maternally derived IgG antibodies to identify potentially protective parasite antigens and indicating the feasibility of using maternal immunization as a means for parasite control.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman H. D. Resistance to anticoccidial drugs in fowl. Parasitol Today. 1993 May;9(5):159–162. doi: 10.1016/0169-4758(93)90137-5. [DOI] [PubMed] [Google Scholar]
- Crane M. S., Goggin B., Pellegrino R. M., Ravino O. J., Lange C., Karkhanis Y. D., Kirk K. E., Chakraborty P. R. Cross-protection against four species of chicken coccidia with a single recombinant antigen. Infect Immun. 1991 Apr;59(4):1271–1277. doi: 10.1128/iai.59.4.1271-1277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lösch U., Schranner I., Wanke R., Jürgens L. The chicken egg, an antibody source. Zentralbl Veterinarmed B. 1986 Oct;33(8):609–619. doi: 10.1111/j.1439-0450.1986.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immunity to coccidiosis: maternal transfer in Eimeria maxima infections. Parasitology. 1972 Oct;65(2):273–282. doi: 10.1017/s0031182000045054. [DOI] [PubMed] [Google Scholar]
- Rose M. E. Immunity to coccidiosis: protective effect of transferred serum in Eimeria maxima infections. Parasitology. 1971 Feb;62(1):11–25. doi: 10.1017/s0031182000071249. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Long P. L. Immunity to coccidiosis: protective effects of transferred serum and cells investigated in chick embryos infected with Eimeria tenella. Parasitology. 1971 Oct;63(2):299–313. doi: 10.1017/s0031182000079610. [DOI] [PubMed] [Google Scholar]
- Wagenbach G. E., Challey J. R., Burns W. C. A method for purifying coccidian oocysts employing clorox and sulfuric acid-dichromate solution. J Parasitol. 1966 Dec;52(6):1222–1222. [PubMed] [Google Scholar]
- Wallach M. G., Mencher D., Yarus S., Pillemer G., Halabi A., Pugatsch T. Eimeria maxima: identification of gametocyte protein antigens. Exp Parasitol. 1989 Jan;68(1):49–56. doi: 10.1016/0014-4894(89)90007-6. [DOI] [PubMed] [Google Scholar]
- Wallach M., Halabi A., Pillemer G., Sar-Shalom O., Mencher D., Gilad M., Bendheim U., Danforth H. D., Augustine P. C. Maternal immunization with gametocyte antigens as a means of providing protective immunity against Eimeria maxima in chickens. Infect Immun. 1992 May;60(5):2036–2039. doi: 10.1128/iai.60.5.2036-2039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


