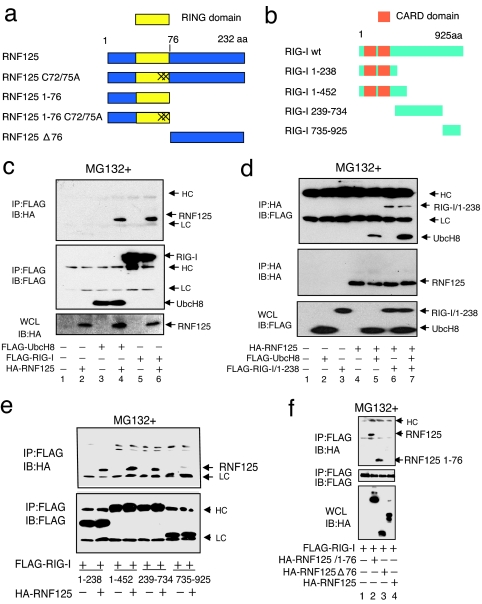
Fig. 1.
Association of RNF125 with UbcH8 and RIG-I. (a and b) Schematic structure of RNF125, RIG-I, and their derivatives used in this work. “X” indicates site of cysteine residue substitution with alanine at the 72nd and 75th residues in RNF125. (c and d) Coimmunoprecipitation experiments. The 293FT cells were transfected as indicated. Thirty-six hours after transfection, protein associations were analyzed either by coimmunoprecipitation using anti-FLAG antibody, followed by Western blot using anti-HA antibody (c), or by coimmunoprecipitation using anti-HA antibody, followed by anti-FLAG antibody (d). (e) Analysis of the RIG-I domain that interacts with RNF125. Plasmids expressing various deletion mutants, including amino acids 1–238, 1–452, 239–734, and 735–925 of RIG-I fused to a FLAG epitope tag (b), were transfected into 293FT cells with or without a plasmid encoding HA-RNF125. Complex formation was examined by immunoprecipitation with an anti-FLAG antibody followed by immunoblotting using the anti-HA antibody (Upper). The amount of each RIG-I mutant in the complex is indicated (Lower). (f) Analysis of the association of RIG-I with RNF125 mutants. Plasmids as indicated were transfected into 293FT cells. The lysates were immunoprecipitated by using an anti-FLAG antibody, followed by blotting with the anti-HA antibody (Top). The quantity of FLAG-RIG-I in the immunocomplexes as well as the RNF125 and mutants in whole-cell lysates are also shown in Middle and Bottom, respectively. HC and LC indicate the heavy and light chains of human immunoglobulins, which are also indicated in the following figures. All cells in c–f were treated with MG132.