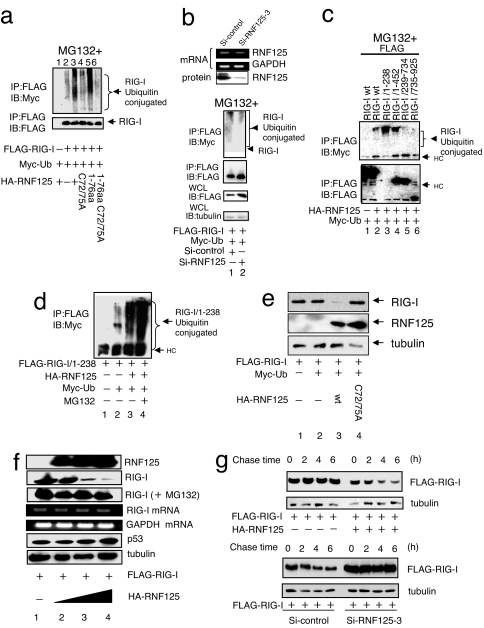
Fig. 2.
Ubiquitin conjugation to RIG-I. (a) Ubiquitin conjugation to RIG-I by WT and mutant RNF125. Plasmids encoding FLAG-RIG-I and Myc-Ub were cotransfected into 292FT cells with a plasmid encoding either WT or mutant RNF125. The RIG-I ubiquitination was monitored by immunoprecipitation. All cells were treated with MG132. (b) Inhibition of RIG-I ubiquitin conjugation after suppression of endogenous RNF125. The siRNF125–3, an siRNA that down-regulates RNF125 mRNA, or a control siRNA were transfected into the 293FT cells. The mRNA and protein levels of RNF125 were monitored by RT-PCR or Western blot, respectively, 36 h after transfection (Upper). The 293FT cells transfected with plasmids encoding FLAG-RIG-I and Myc-Ub were simultaneously treated with control siRNA or siRNF125–3; RIG-I ubiquitination in cell lysates was then analyzed by coimmunoprecipitation. All cells were treated with MG132. The quantity of RIG-I present in the immunoprecipitants was monitored by immunoblot with an anti-FLAG antibody. The total RIG-I present in an aliquot of whole-cell lysate is also shown. Tubulin serves as the control in this analysis (Lower). (c) Analysis of the region for ubiquitin conjugation in RIG-I. Plasmids encoding HA-RNF125 and Myc-Ub were cotransfected into 293FT cells with WT or deletion mutants of FLAG-RIG-I. We analyzed cell lysates harvested 36 h after transfection for interactions between RNF125 and WT or mutant RIG-I by immunoprecipitation (note that the expression level of 1–238 was very low; despite the low levels, however, the interaction with RIG-I was clearly observed). All cells were treated with MG132. (d) Ubiquitin conjugation stimulates RIG-I degradation in a proteasome-dependent manner. Plasmids encoding a FLAG-tagged version of the RIG-I N terminus, FLAG-RIG-I/1–238, HA-RNF125, and Myc-Ub were transfected into 293FT cells as indicated. After culturing with or without MG132, the levels of ubiquitination were analyzed by immunoprecipitation. (e) RNF125 stimulates RIG-I degradation. Plasmids encoding FLAG-RIG-I and Myc-Ub were cotransfected into 293FT cells with WT or a point mutant of HA-RNF125. Cells were harvested 36 h after transfection and were analyzed for proteins by Western blot. (f) RIG-I was degraded by RNF125 in a dose-dependent manner. The 293FT cells were transfected with a plasmid encoding FLAG-RIG-I (0.5 μg) and varying doses of a plasmid encoding HA-RNF125 (0, 0.5, 1, and 2 μg). Half of each cell aliquot was treated with MG132. The levels of both RIG-I mRNA and protein were examined in cells harvested 48 h after transfection. “RIG-I (+MG132)” indicates analysis of the RIG-I protein in cells treated with MG132. As a control, the cellular proteins p53 and tubulin and GAPDH mRNA analyses are shown. (g) RIG-I was degraded by ectopic and endogenous RNF125. A plasmid encoding FLAG-RIG-I with or without a plasmid encoding HA-RNF125 was transfected into 293FT cells. Thirty-six hours after transfection, the cells were treated with CHX (final concentration, 50 μg/ml), and were analyzed by Western blot (Upper). Plasmids encoding FLAG-RIG-I and RNA for si-control or siRNF125–3 were transfected into 293FT cells. Twenty-four hours after transfection, these cells were treated with Poly I:C for 12 h, followed by addition of CHX. Cells were harvested at the indicated times after addition of CHX and analyzed by Western blot (Lower).