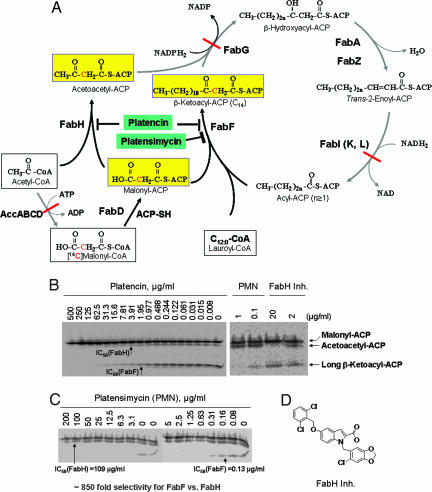
Fig. 3.
Determination of target selectivity using FabH/FabF PAGE elongation assay. (A) Schematic description of FabH/FabF PAGE elongation assay. Bacterial fatty acid biosynthesis was initiated with acetyl-CoA, which is carboxylated by AccABCD in the presence of ATP to form malonyl-CoA, which is, in turn, transferred to ACP by FabD. Fatty acid synthesis is initiated by FabH supplying substrates (acetoacetyl-ACP) to the fatty acid elongation cycle, which includes FabG, FabA/Z, FabI (L/K), and FabF/B enzymes. In the cycle, the keto group of β-ketoacyl-ACP is reduced to a hydroxyl group by NADPH2-dependent reductase FabG. β-Hydroxyacyl-ACP is dehydrated by dehydratase FabA or FabZ. The double bond of trans2-enoyl-ACP is reduced by NADPH2-dependent reductase FabI(K/L), which feeds the substrate back to FabF/B, which in turn adds an additional acetate unit (two carbons), and the cycle iterates. When the assay is performed with all natural enzymes without addition of ATP and NADPH2/NADH2, both AccABCD reaction and the elongation cycle are unable to proceed. By addition of radiolabeled malonyl-CoA and lauryl-CoA, as well as the natural substrate acetyl-CoA, only three radiolabeled protein (ACP) products (shown in yellow boxes) are obtained: malonyl-ACP (FabD product), acetoacetyl-ACP (FabH products), and long-chain β-ketoacyl-ACP (C14, FabF products). These products can be separated by urea PAGE (26). (B and C) FabH/FabF PAGE elongation assay was performed as described in Materials and Methods. Three substrates, acetyl-CoA, lauryl-CoA (C12:0), and [14C]malonyl-CoA, were added without addition of ATP, NADH2, and NADPH2, and only three products were obtained: malonyl-ACP (FabD product), acetoacetyl-ACP (FabH product), and long β-ketoacyl-ACP (C14, FabF product). These products can be separated with 0.5 M urea PAGE. (B) A FabH inhibitor (FabH Inh.) did not inhibit FabF activity but blocked FabH activity with 90% and 50% inhibition of the accumulation of acetoacetyl-ACP at 20 and 2 μg/ml, respectively, whereas platensimycin selectively inhibited FabF and accumulated long-chain β-ketoacyl-ACP without inhibition of FabH activity. In contrast, platencin (tested with a serial dilution) inhibited both FabF and FabH, providing IC50 values of 1.95 and 3.91 μg/ml, respectively. (C) The assay was performed with a serial dilution of platensimycin, giving an 838-fold selectivity for FabF over FabH. The IC50 values are 0.13 μg/ml for FabF and 109 μg/ml for FabH. Both platencin and platensimycin did not show any inhibition against FabD protein. (D) Chemical structures of FabH inhibitor (FabH Inh.).