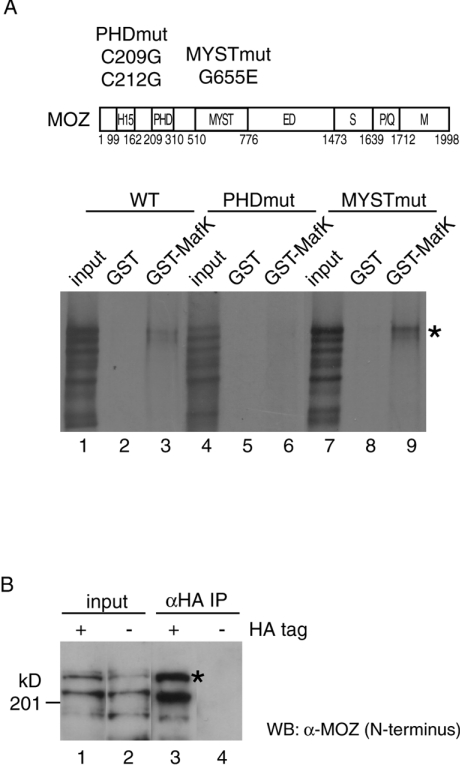
Figure 4. MOZ interacts with MafK in vitro and in vivo.
(A) Structural domains of MOZ were indicated as follows: H15, histones H1- and H5-like module; MYST, MYST acetyltransferase domain; ED, glutamic acid/aspartic acid-rich acidic regions; S, serine-rich domain; P/Q, proline/glutamine-stretch; and M, methionine-rich domain. Also shown are the mutation positions in the PHD finger and MYST regions. Indicated wild-type and mutated in vitro-translated [35S]MOZ proteins were incubated with GST (lanes 2, 5 and 8) or GST–MafK (lanes 3, 6 and 9). MOZ protein retained on the GST-conjugated beads after extensive washing was analysed by SDS/PAGE and autoradiography. The amount of input (lanes 1, 4 and 7) is equivalent to 10% of the reaction volume in the assay. [35S]MOZ proteins are indicated by asterisks (*). (B) MOZ expression plasmid was co-transfected with HA-tagged MafK (lanes 1 and 3) or nontagged MafK (lanes 2 and 4) into HeLa cells, and nuclear extracts were prepared. Immunoprecipitation (IP) experiments were performed with anti-HA antibody. Immunoprecipitates (lanes 3 and 4) and 5% of input (lanes 1 and 2) were resolved by SDS/PAGE (7.5% gel) and detected by Western blotting using anti-N-terminal MOZ antibody. MOZ proteins are indicated by asterisks (*).